Advances in Animal and Veterinary Sciences
Research Article
Effect of Smoke Duration on Compositional Analysis, Deterioration Criteria, Microbial Profile and Sensory Attributes of Marine and Freshwater Fish: A Comparative Study
Hamdy M.B.A. Zaki*, Mohamed M.T. Emara, Marwa R.S. Abdallah
Department of Food Hygiene and Control, Faculty of Veterinary Medicine, Cairo University, Giza square, Giza 12211, Egypt.
Abstract | Smoked fish is considered one of the most commonly consumed aquatic food. The impact of fish smoking technology on the quality criteria varies depending on the smoking time and/or temperature as well as the type of fish used. This study focused on studying the effect of using three different hot smoking durations (30, 45, and 60 min.) on different quality attributes of both marine fish (Epinephelus marginatus) and freshwater fish (Lates niloticus) during chilled storage (4ºC) under vacuum packaging for three months. Results revealed that 60 min. smoking of fish fillet achieved the best quality results as it increased the phenolic substances that resulted in a marked decline in the lipid oxidation criteria. Furthermore, increasing the smoke duration significantly decreased the microbial count of the total anaerobic sporeformers, Staph aureus count, and mold counts to below the detectable limit (<2 log10 CFU/g) while the aerobic plate count reached 2 log10 CFU/g by 60 min smoking in both fishes. Moreover, protein and salt content increased by increasing smoking time, while moisture and the available water (aw) content decreased. Sensory evaluation revealed that 45 min. and 60 min. smoking had the best sensory score compared to 30 min. smoking. All measured attributes increased gradually during chilled vacuum storage.
Keywords | Smoked fish, Hot smoking technology, Lipid oxidation, Microbial profile, Proximate analysis
Received | April 28, 2021; Accepted | June 02, 2021; Published | July 15, 2021
*Correspondence | Hamdy MBA Zaki, Department of Food Hygiene and Control, Faculty of Veterinary Medicine, Cairo University, Giza square, Giza 12211, Egypt; Email: hamdy.bakry@vet.cu.edu.eg
Citation | Zaki HMBA, Emara MMT, Abdallah MRS (2021). Effect of smoke duration on compositional analysis, deterioration criteria, microbial profile and sensory attributes of marine and freshwater fish: a comparative study. Adv. Anim. Vet. Sci. 9(8): 1259-1266.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.8.1259.1266
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Zaki et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Fish is considered an excellent food of high-quality protein that can replace both red and white meat especially with the fast-growing aquaculture industry in recent years (Kari et al., 2020). It contains both important micro- (minerals and vitamins) and macro- (protein, fat) nutrients. Furthermore, fish contains a high level of polyunsaturated fats (PUFA) which helps in lowering cardiovascular diseases in humans (Mishra, 2020). However, fish is considered a highly perishable food that undergoes many deteriorative changes including propagation of bacterial flora as well as lipid oxidation, enzymatic and chemical changes which led to rapid spoilage (Khoshmanesh, 2006).
Different preservation techniques e.g., cold storage, salting, drying, fermentation, and smoking are commonly used in fish preservation technology (Alcicek and Atar, 2010). For centuries, smoking is considered the chief method of fish preservation and is still used worldwide in many countries (Bilgin et al., 2008). During smoking, several compounds e.g., phenols and formaldehyde result from the partial burning of specific types of wood. The smoke products impart a harmful effect on both spoilage and food poisoning bacteria and retard the oxidative enzymes which collectively preserve the fish quality and extend its shelf life (Pagu et al., 2013). Moreover, smoking increases the protein content and reduces moisture content, it also resulted in value-added products by improving the sensory acceptability of fish (Akinwumi, 2014).
Many processors prefer the use of smoking as an easy and simple method for fish preservation to elongate its shelf life and allows selling it at a higher price (Magawata and Musa, 2015). However, most processors thaw fish before smoking, ignoring the possibility of microbial proliferation under uncontrolled thawing conditions. The technology of fish smoking is a series of steps that begin with salting, drying then smoking under controlled temperature e.g., below 30 ºC (cold smoking) (Montero et al., 2007), and at 52 to 85ºC (hot smoking). Unfortunately, the cold smoking technique is not adequate to inactivate bacteria and consequently may carry a risk of high bacterial load including many food poisoning microbes (Vaz-Velho et al., 2006). However, the temperature of hot smoking is enough to halt microbial growth as well as the destruction of the enzymes responsible for oxidation (Arvanitoyannis and Kotsanopoulos, 2012), because the thermal process lowers the available water to a level that prevents microbial growth. Moreover, the density of smoke, the concentration of its active components in combination with the salt content, and the time and temperature of smoking all influence the spoilage and pathogenic microflora of smoked fish (Kolodziejska et al., 2002).
Egypt has a diverse range of water sources including both marine and freshwater which led to the availability of vast amounts of fish estimated at 1.61 million tons in 2016 (Hassan et al., 2019). Post-harvesting losses due to the rapid decomposition prove the need for use of a reliable preservation method to improve the quality and shelf life of the fish. Consequently, the current study planned to use three different hot smoking times to assess the best method having a good impact on the sensory, physicochemical, and microbial quality parameters of one marine and one freshwater fishes available in the Egyptian market.
Materials and methods
Experimental design for fish processing
Two fish types one marine (Epinephelus marginatus) and the other freshwater (Lates niloticus) were used in this experiment. A total of 100 kg (50 of each) was collected from Al-Aboor Fish Market, Cairo, Egypt during December 2019. The fish was stored in crushed ice and transported to the Development and Researches unit in a main food processing plant. Immediately after arrival, the fish were cleaned, carefully washed, and eviscerated with the removal of both the skin and bone and finally cut into uniform pieces of about 100 g each under good hygienic practices. The fish fillets were soaked in 10% salt solution for 2 hr at 4°C (1:2 w/v fish to brine). After salting, the fillets were rinsed in water and divided into 3 groups for each fish type, and each group was subjected to a different hot smoking program.
A Maurer-Atmos oven equipped with a smoke generator in which Beechwood (Günter Springer Spanholz GMBG & Co. Kg, Germany) was burned to produce smoke was used for application of the smoke. The salted fish fillets were arranged on a perforated surface pre-greased with sunflower oil on smoking trays to prevent sticking to the sheet. The fillets were first dried for 2hr at 50°C, then smoked to either 30, 45, or 60 min with a smoking temperature of 70 °C (50% smoke/50% air). After that, the fish fillets were left to cool, stored at 4°C for the next day, and finally vacuum packed (200±10.00 g portions) in a clear transparent Nylon LLDPE Co-ex Low-Density Polyethylene vacuum storage packaging bags (Vollrath Company, USA) and vacuum sealed using Komet SD520 double chamber vacuum packing machine (KOMET MASCHINENFABRIK GMBH, Germany) at a pressure of 0.8 bars for 30 seconds. The plastic film in the vacuum packaging bags was 40 microns thick, with an oxygen transfer rate of 3.99 cc/100 in2/24hr (65% RH, 23 °C), and moisture vapor transfer rate of 0.54 g/100in2/24 hr. (90% RH, 38 °C). After packaging, all packages were stored at 4°C and the samples were drawn the next day and monthly for sensory, physicochemical, chemical, and microbial analysis.
Examination of smoked fish
Proximate chemical examinations
Each sample was minced three times and mixed after each time before used for the proximate chemical analysis following the Guidelines of AOAC (2005).
Physicochemical examinations
Measurement of total phenols: Four grams of sample were homogenized for 1 min with 50 ml ethanol (95%) then centrifuged for 10 min at 2500g. Five ml of each sample supernatant was mixed with 30 ml distilled water and 0.6 ml phenyl-2,3-dimethyl-4-amino5-pyrazolone solution (2%) in a decantation flask, then 2 ml 2 N ammonia solution were added. After thorough mixing, 2 ml potassium hexacyanoferrate solution (2%) was added. The mixture was left for 5 min before 10 ml chloroform was added and stirred for 15 min. The chloroform phase was then filtered using No. 126 Durieux filter contained 3 g anhydrous sodium sulphate and the optical density was read at 455 nm using Unico 1200 series spectrophotometer against a standard curve prepared from decimal dilutions of 1 mg/l standard phenol solution (Cardinal et al., 2004).
Determination of pH, thiobarbituric acid reactive substances (TBARS) values: Five grams sample was stirred in 20 ml distilled water for 1 min and used for measurement of pH using a digital pH meter (Lovibond Senso Direct) equipped with a probe-type combined electrode (Senso Direct Type 330). The distillation method of Torres-Arreola et al. (2007) was used to determine the TBARS content.
Determination of peroxide value (PV): Five g prepared fish samples were mixed thoroughly with 30 mL of chloroform/glacial acetic acid (1:3) and 0.5 ml saturated potassium iodide then left for 1 min in a dark place with occasional spinning then 30 mL distilled water was added. The mixture was titrated with 0.1 N sodium thiosulphate solution with 1 mL of 1.0% soluble starch. A blank titration was performed following the same steps without a sample (AOAC, 2005). The PV (milliequivalents peroxide/1000 g sample) was calculated using the equation of Latip et al. (2014).
Determination of total volatile basic nitrogen (TVBN) and salt content: Total volatile basic nitrogen was measured by the method outlined by (Antonacopoulos and Vyncke, 1989). Salt concentrations expressed as percentage were determined according to (AOAC, 2005).
Determination of Water activity (aw): The water activity of both fresh and smoked fish fillet was measured using an Aqualab 4TE (Meter Group, Inc, USA). Two grams sample were put in the water activity meter and three readings of aw for each replicate were automatically measured and the mean was calculated.
Evaluation of microbiological quality
Smoked fish sample preparation and tenfold decimal dilutions were according to ISO/6887-1 (2017). The assessment of the microbial quality includes enumeration of the total aerobic plate count using plate count agar (Oxoid CM0325) and incubation at 35 °C for 48 hr (Dale Morton, 2001), the total mesophilic anaerobic sporeformers bacterial count using Reinforced Clostridial Agar “RCM” (Oxoid CM0149) and anaerobic incubation in a gas pack system at 35 ºC for 48 h (Scott et al., 2001). Incubation of Baird-Parker agar plates (Oxoid, CM1127) at 37 ºC for 48 hours was used for enumeration of the presumptive Staph. count (Bennett and Ga, 2016), while incubation of Sabaroud dextrose agar (M063 HIMEDIA, Germany) for 5 days at 25 ºC was used for counting the total mold counts (Beuchat and Cousin, 2001). The average count for each sample was reported as log10 Colony-Forming Units/g sample (log10 CFU/g).
Sensory evaluation
Smoked E. marginatus and L. niloticus fillet were sensory evaluated using a 9-points hedonic scale (1 denotes unacceptable and 9 denotes highly acceptable) and the method described by (AMSA, 2015). Sensory evaluation was conducted by 10 panelists from the staff members, workers, and students of the Department of Food Hygiene and Control, Faculty of Veterinary Medicine, Cairo University, Egypt. Panelists were from both sexes and with variable ages (20–40 years). Before the main sensory evaluation session, the panel team was extensively trained to be familiar with the variations in smoked color, smoked flavor, texture, and overall acceptability of smoked fish fillet. The sensory analysis session was repeated 2 times at different times and the average of the sensory panel scores for each parameter was calculated as a final result.
Data statical analysis
Statistical analysis for data was carried out using SPSS statistics 23.0 program for windows. The experiment was performed in three replicates and all examined parameters were measured three times for each replicate, then the average values of the obtained data were expressed as mean ± standard error (SE). Regarding microbiological analysis, the data was first converted from normal count (CFU/g) into a logarithmic value (log10 CFU/g). The data of each type of fish were compared during storage time using one-way analysis of variance (ANOVA). Moreover, the Independent Sample T-test was used to compare the results between the two fish types in raw fish examinations. Significances were determined by using the least square difference test (LSD) procedure. Differences were considered significant at the P < 0.05 level.
Results and Discussion
The quality characteristics of raw fishes (Table 1) revealed that the non-significant differences in fat, protein, ash, water activity content among investigated fish samples, the moisture content of E. marginatus was significantly higher than that of L. niloticus. Assessment of the fat oxidation criteria (TBARS, PV) and the indicator of freshness (pH, TVBN) showed that the raw fish samples used in this experiment were of good quality, with E. marginatus having substantially higher values than L. niloticus. In contrast to E. marginatus, APC, Staph aureus, and total mold counts were significantly lower in L. niloticus. Meanwhile, the anaerobic mesophilic sporeformers counts of both fishes were not significantly different.
Hot smoking had a major impact on the chemical composition of both fishes immediately after processing and throughout their storage life (Table 2 and Fig. 1). In both species, hot smoking resulted in a fundamental and time-
Table 1: Quality of raw fish used for different methods of hot smoking
| E. marginatus | L. niloticus | |
| Proximate chemical analysis (g%) | ||
| Moisture |
77.26±1.22a |
76.90±0.09b |
| Protein |
20.08±0.56a |
19.85±0.25a |
| Fat |
1.15±0.08a |
1.22±0.10a |
| Ash |
1.19±0.05a |
0.92±0.01a |
| Physicochemical criteria | ||
| pH |
6.86±0.55a |
6.60±0.43b |
| TVBN (g/100g) |
9.27±0.80a |
8.50±0.77a |
| TBARS (g/Kg) |
0.43±0.90a |
0.24±0.02b |
| PV (meq/Kg) |
7.25±1.20a |
4.24±0.92b |
|
aw |
0.99±00a |
0.98±0.001a |
|
Microbiological load (Log10 CFU/g) |
||
| APC |
5.43±0.38a |
4.81±0.27b |
| Total Anaerobic sporeformers count |
3.66±0.32a |
3.20±0.29a |
| Staph. aureus |
4.34±0.41a |
3.69±0.18b |
| Total Mold |
4.43±0.29a |
3.91±0.16b |
Means of three replicates in three separate fishes each
a-b Means with different superscripts differ significantly between the two fishes at P< 0.05
Table 2: Effect of hot smoke duration on the quality criteria of E. marginatus and L. niloticus
| E. marginatus | L. niloticus | |||||
| 30 min. | 45 min. | 60 min. | 30 min. | 45 min. | 60 min. | |
| Proximate chemical analysis (g%) | ||||||
| Moisture |
67.24±2.34a |
62.59±1.55b |
57.28±1.96c |
66.90±1.89a |
60.20±1.65d |
56.01±1.23c |
| Protein |
24.30±1.98a |
27.50±2.00b |
28.90±2.14c |
23.00±1.67a |
25.99±1.99d |
26.11±2.04b |
| Fat |
1.41±0.3a |
1.72±0.5b |
2.02±0.1c |
1.53±0.08d |
1.76±0.06b |
1.98±0.10c |
| Ash |
6.55±0.25a |
7.98±0.33b |
8.35±0.41b |
6.12±0.19a |
7.53±0.27b |
8.10±0.43c |
| Physicochemical criteria | ||||||
| Phenol ppm |
44.95±3.29a |
55.34±2.87b |
70.54±3.38c |
45.33±2.76a |
57.96±3.45b |
74.23±2.65d |
| TBARS (g/Kg) |
0.76±0.09ac |
0.70±0.11ab |
0.66±0.20b |
0.85±0.12c |
0.70±0.22ab |
0.57±0.31d |
| PV (meq/Kg) |
12.55±0.89a |
10.2±10.56b |
9.10±1.08c |
9.89±0.54b |
8.50±0.43c |
6.37±0.62d |
| pH |
6.60±0.50a |
6.02±0.45b |
5.75±0.55c |
6.44±.0.45a |
5.86±0.40c |
5.77±0.49c |
| TVBN (g/100g) |
10.20±1.10a |
9.10±1.22b |
8.38±0.98bc |
9.19±1.11b |
8.30±1.90c |
8.00±0.99c |
|
aw |
0.919±0.01a |
0.826±0.04b |
0.635±0.01c |
0.907±0.02a |
0.808±0.01b |
0.619±0.03c |
| Salt g% |
2.90±0.11a |
3.45±0.32b |
4.42±0.29c |
2.82±0.17a |
3.30±0.15b |
4.15±0.42c |
|
Microbiological load (Log10 CFU/g) |
||||||
| APC |
2.77±0.14a |
2.68±0.21a |
2.00±0.20b |
2.85±0.16a |
2.70±0.16a |
2.00±0.11b |
| Total Anaerobic sporeformers count |
2.75±0.22a |
2.00±1.00b |
<2.00±0.00c |
2.90±0.19a |
2.00±0.27b |
<2.00±0.00c |
| Staph. aureus |
<2.00±0.00a |
<2.00±0.00a |
<2.00±0.00a |
<2.00±0.00a |
<2.00±0.00a |
<2.00±0.00a |
| Total Mold |
3.00±0.20a |
<2.00±0.00b |
<2.00±0.00b |
2.00±2.00c |
<2.00±0.00b |
<2.00±0.00b |
a-d Means with different superscripts in the same row differ significantly at P< 0.05.
dependent increase in the important macronutrient component (protein, fat) and a decrease in moisture probably due to dehydration (Salán et al., 2006). The increased duration of smoking resulted in moisture declined from about 77% in fresh fillet to 66.90-67.24% after 30 min. smoking to reach the lowest significant value (57.28-56.0.1%) after 60 min. smoking compared to 30 min. This decrease in moisture content may be attributed to dryness prior to
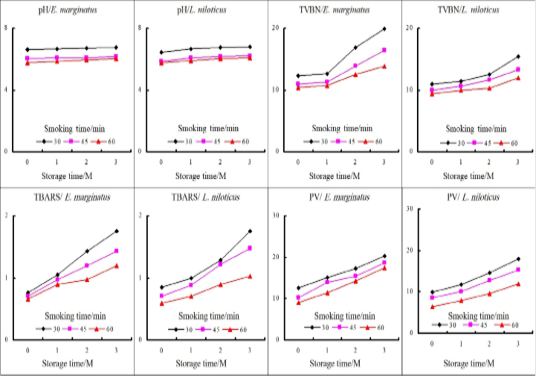
Figure 1: Changes in the physicochemical criteria of vacuum-packed hot smoked fish during storage at 4C.
smoking, as well as the high temperature exerted by the duration (30-60 min) and smoking temperature (70 ºC). These findings were consistent with those of Adeyemi et al. (2013) and Sulieman et al. (2018), who found that hot smoking reduced moisture content while increased the protein content in smoked fish fillets. The smoked fillets had higher protein contents than fresh samples, with the highest protein values observed after 60 minutes of smoking (28.9% in E. marginatus and 26.11% in L. niloticus), which could be clarified by the inverse relationship between moisture vs protein and fat. Similar results for the chemical composition of smoked fish have been reported by Okerreke et. al. (2014) who found the increase in protein content of smoked Clarias gariepinus was due to loss of moisture and concomitant protein concentration during the thermal processing. Moreover, Akintola (2015) found that the smoking time exerted a time-dependant effect on moisture content and aw which increased the shelf span of smoked fish.
Total phenol derivatives are generally extracted from the thermal degradation of wood. These fractions are important due to their antioxidant and antimicrobial properties as well as the sensory attributes of smoked food products (Kjällstrand and Petersson, 2001). Total phenolic compounds increased as smoking time increased with mean values ranged from about 50 to 74.23 ppp, moreover, the values for L. niloticus being significantly higher than E. margina values if the smoke duration reached 60 min.
The food safety agencies recommended the assessment of TBARS, PV, TVBN, and pH values as they threatening the quality, shelf life, and acceptability of smoked fish (El-Lahamy et al., 2019). The results revealed an increased level of TBARS up to 2-3 folds in smoked fish compared to raw fish. Goktepe and Moody (1998) observed a two-fold increase in TBA value of raw Catfish after hot smoking. The data also showed a steady decline in both TBARS and PV levels as a result of increasing the time of smoking in both fishes (Table 2). Muratore et al. (2007) established that extending the time of smoking increased the deposition of phenolic antioxidant compounds on the surface of fish fillet which ultimately retard the rate of rancidity and extend the product shelf life. During vacuum packing storage, both TBARS and PV linearly increased from the 1st month of storage to reach unacceptable limits at the end of the storage trial (Figure 1). TBARS values for fish products below 0.58 mg/kg are considered not rancid, 0.58–1.51 mg/kg are slightly rancid but acceptable, and values above 1.51 mg/kg are considered rancid (Ke et al., 1984). In this case, all samples were deemed acceptable except the first treatment at the third month of storage. Concerning the peroxide value, all samples were acceptable according to Daramola et al. (2007) who reported that fish is considered spoiled at 20-40 (ml per Kg).
Smoking resulted in a steady decline in pH values in comparison with the fresh fishes. Moreover, increasing the time of smoking was correlated with a further linear decline in pH values (Table 2). The decrease in pH values may be due to the addition of common salt during the marination process which increased the ionic strength. Moreover, the deposition of organic acids e.g., acetic acid during the smoking process also resulted in a further decline in the pH value of smoked fish (Toledo, 2008). On the other hand, the pH values started to elevate gradually to reach to its highest limit of (<6.60) starting from the 1st month of the storage period in the 1st treatment, however, longer smoking time preserved the pH within lower limits through the storage trial in both types of fish (Figure 1).
Both the cellular and microbial enzymes break down proteins into volatile nitrogenous compounds resulted in the elevation of pH and TVBN. However, it was noticed that increasing the smoking duration decreased the TVBN contents for each fish (from 10.2 to 8.83 in E. marginatus and from 9.19 to 8 in L. niloticus) as showed in Table 2. Longer smoking time resulted in a more destructive effect on both endogenous and microbial enzymes that consequently lower the protein breakdown and TVBN (El-Lahamy et al., 2019). The TVBN values increased significantly during the storage period (Figure 1) but still within the acceptable limit (30 mg/100g) reported by Daramola et al. (2007). The cause of the increase in TVBN at the end of storage time may be probably due to bacterial spoilage and bacterial decarboxylation (Castro et al., 2012).
Data in (Table 2) clarify the reduction of moisture content by increasing the smoking time corresponded to a significant increase in salt content and a significant decrease in (aw) value. Removal of water during the smoking process and the diffusion of salt during the brining process caused a time-dependant rise in salt content. The mean (aw) percentage of the raw fish fillet in both fishes was about 1.000 (Table 1), and its value was decreased with increasing the duration of smoking in both fishes while L. niloticus had significantly lower values than E. marginatus. The obtained results were in agreement with Oyero (2006) who stated that (aw) is directly proportional to moisture content and in turn influenced the microbial and oxidative stability of foods.

Figure 2: Changes in microbial load (log10 CFU/g) of vacuum-packed hot smoked fish during storage at 4C.
Microbial analysis of raw fish fillet revealed a reasonably high level of all investigated microbial groups where total aerobic bacteria showed the highest level in both fishes followed by Staph. aureus while molds showed a fairly high count (Table 1). Fresh fish with a high total bacterial count would deteriorate quickly, and the presence of staphylococci suggested improper handling, while high mold count may pose human health hazards due to production of mycotoxins which necessitating careful treatment and preservation to maintain nutrients and functional components that promote good health. In general, all of the smoking programs used in this study reduced the microbial load in smoked fish fillets as compared to raw fish fillets. Staph. aureus counts were reduced to below the detectable limit (2 log10 CFU/g) in all of the smoking times. Furthermore, raising the smoking time resulted in a substantial decrease in the values of the other microbial populations (Table 2, Figure 1 & 2). In general, increasing the smoking period gradually decreased the microbial load for example the 60 min. smoking had the most obvious destructive effect on all microbial counts compared to 30 min smoking in both fishes at zero time and throughout the storage period (Table 2, Fig. 2). The reduction in microbial load could be owed to several hurdle factors e.g., decrease in pH, reduced (aw), long exposure to high temperature (70 ºC/60 min.), increase the salt content, and the bactericidal and antioxidant effect of phenolic content which increases with the time of smoking (Table 2, Figure 1 & 2). Dutta et al. (2018) reported that almost all microbes are killed by hot smoking, except certain pathogenic bacteria, since the fish is cooked and dried at high temperatures. According to Leroi et al. (2000), total aerobic bacteria were inhibited primarily by salt content and to a lesser degree by phenol content and this inhibition was proportional to the amount of salt and smoke present. Finally, the study pointed out that although smoking inhibited the microbial activities, the microbial growth continued during chilling storage till it reached unacceptable levels by the end of the 3rd month, causing the fish to deteriorate in terms of high pH, TBARS, PV, and TVBN.
The examined sensory attributes varied among different smoking methods where the 45 min. smoked fish fillet had the best sensory score followed by smoking for 60 min. Meanwhile, the lowest significant sensory score was obtained after 30 min. of smoking. It was also obvious that the consumers preferred moderate and intense smoking techniques rather than short-time smoking techniques, and increasing the smoking time of fish fillet positively affects its acceptability (Fig. 3). The obtained results also revealed that under the same smoking conditions, E. marginatus smoked fish fillets achieved a better sensorial acceptance than L. niloticus. Moreover, chilled (4ºC) storage had little impact on all sensory attributes. The variation in color score may be attributed to the effect of fat oxidation, as well as the production of high amounts of peroxides during smoking (Olukayode and Paulina, 2017). As a result, consumers favored moderately smoked fish over long-smoked fish. The difference in sensory color scores between the two fishes may be attributed to differences in the rate of both protein and fat decomposition caused by heat treatment, as well as oxidation of polyunsaturated fatty acids at high temperatures (Abdul-Baten et al., 2020). The difference in flavor scores may be explained based on the microbial decomposition of nutrients during storage (Famurewa et al., 2017) due to the absorption of water that occurred between salted smoked fish and humidity, which increased aw and allowed the decomposition of proteins and fats to amines, ammonia, peroxides, and free fatty acids, which result in off-flavor and rancid taste (Pal et al., 2016). The differences in texture may be due to variations in moisture loss during the brining, drying, and application of smoke (Jakhar et al., 2015), while the variation in overall acceptability of smoked fish could be due to the technology’s effectiveness in removing water, which affects the visual and nutritive nature of smoked fish (Abdul-Baten et al., 2020).
Conclusion
From this study, it could be concluded that increasing the hot smoking duration to 60 min. has a positive effect on both marine and freshwater fishes. Smoking fish fillet to 60 min. improved the antimicrobial, nutritional, antioxidation as well as sensory criteria. Smoking fish fillet to 45 min. have a less favorable impact than 60 min. meanwhile 30 min. smoking had the lowest desirable effect. It is recommended to use a combined time/temperature hot smoking technique of 70 °C/60 min. to gain the best quality criteria of smoked fish fillet for both fresh and marine fishes. Chilled storage maintained the different quality attributes within the acceptable limits. Using alternative storage conditions for smoked fish fillets’ prolonged preservation could be an interesting point for further studies.
Acknowledgments
Authors acknowledge Cairo university.
Conflict of interest
Authors declare that there is no conflict of interest.
Author’s contribution
Authors shared an equal contribution in this manuscript.
References






