Advances in Animal and Veterinary Sciences
Research Article
The Immunological Effect and Pharmacological Interaction of Tylvalosin with Lactobacillus acidophilus During Vaccination of Chickens with NDV Vaccine
Mai A. Fadel1*, Maha S. Abd El-Hafeez1, Mahmoud Mohamed Abd El-Moneam2
1Department of Chemistry, Toxicology and Feed Deficiency, Animal Health Research Institute (AHRI), Agricultural Research Center (ARC), Dokki, Giza, Egypt; 2Department of Newcastle Disease Vaccine Research, Veterinary Serum and Vaccine Research Institute (VSVRI), Agricultural Research Center (ARC), Cairo, Egypt.
Abstract | Antibiotics administration to chicks is common for metaphylaxis or treatment of suspected infections. Tylvalosin is a macrolide antibiotic with an antiviral activity. Through this study, we highlighted tylvalosin role on broiler’s immune response after Newcastle disease virus (NDV) vaccination when administrated alone or together with a probiotic bacterium Lactobacillus acidophilus. Immune response was measured by challenge, hemagglutination inhibition (HI) tests and interleukin 8 (IL-8) level. Moreover, the study evaluated pharmacokinetics and tissue residues of tylvalosin by a modified validated HPLC method when administrated alone or with ND vaccination and/or L. acidophilus intake. The results illustrated that both NDV vaccinated group (V) and NDV vaccinated group treated with tylvalosin (VT) showed full (100%) protection in response to challenge with very virulent NDV. The non-vaccinated groups (T, L) showed 66.6% and 40%, respectively. Besides, (VT) group had no significant change in HI titer and IL-8 concentration if compared with (V) group. Pharmacokinetic parameters of (VT) group revealed significant decrease in (area under curve AUC (0-t, 0-info) with Cmax) and significant increase in (t0.5β, V/F, CL/F and Tmax than group treated with tylvalosin alone (T) and vaccinated group treated with tylvalosin and L. acidophilus (VTL). The highest residues were detected in lung and liver and the lowest in muscle. The recommended withdrawal time in VT group was 4 days but in groups (T & VTL) was 5 days. Finally, tylvalosin has a synergistic action with NDV vaccine as it has no negative effect on the immunity to NDV vaccine which enhances significantly tylvalosin pharmacokinetics and residues. L. acidophilus didn’t cause any significant amelioration for poultry.
Keywords | Tylvalosin- NDV- Lactobacillus acidophilus - Kinetics-residues.
Received | April 08, 2021; Accepted | April 30, 2021; Published | July 15, 2021
*Correspondence | Mai A Fadel; Researcher of Pharmacology, Department of Chemistry, Toxicology and Feed Deficiency, Animal Health Research Institute (AHRI), Agricultural Research Center (ARC), Giza, Egypt; Email: dr.mai87@yahoo.com
Citation | Fadel M.A., Abd El-Hafeez M. S, Abd El-Moneam M.M (2021). The immunological effect and pharmacological interaction of tylvalosin with lactobacillus acidophilus during vaccination of chickens with NDV vaccine. Adv. Anim. Vet. Sci. 9(8): 1249-1258.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.8.1249.1258
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Fadel et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Newcastle disease (ND) is a highly contagious viral disease of poultry that is characterized by respiratory, nervous, enteric, and reproductive symptoms, caused by virulent ND virus (vNDV), which belongs to the genus Avulavirus within the family Paramyxoviridae (Alexander and Senne, 2008; Liu et al., 2016). ND has been a devastating disease and it remains one of the major problems in existing and developing poultry industries in many countries Arifin et al. (2010). There are no treatments available for ND; so, vaccination is an effective control method. Live-virus vaccines for ND may be administered by drinking water, aerosol or eye drops. Infection occurs on or through mucosal surfaces; the majority of vaccines are administered parenterally Henderson et al. (2011).
The most efficient way to combat diseases is to elevate the immunity then using effective drugs for achieving the rapid recovery. The use of some probiotics conserves a good balance for beneficial microflora in the chicken gut which provides an improvement of general immunity and health (Callaway et al., 2008; Gonmei et al., 2019). Lactobacilli are nonpathogenic Gram-positive inhabitants of intestinal microbiota that are widely used as probiotics Awad et al. (2009). In chicken, treatments with various members of the Lactobacillus species (L. acidophilus, L. reuteri, and L. salivarius) have been shown to stimulate both innate and adaptive immune response through the ability to modulate chicken cytokine which promote T-helper 1(Th-1) such as interleukin 12 (IL12) and chemokine gene expression Brisbin et al. (2010) and Brisbin et al. (2008), enhance the expression of Toll-like receptor (TLR) and T cell-related mRNA expression levels in the gut Sato et al. (2009), enhance the function of T cells in newly hatched chicks, increase the number of intestinal epithelial lymphocytes (IELs) expressing CD3, CD4, CD8, and T cell receptor (TCR) αβ, and improve systemic antibody response. (Forsythe and Bienenstock, 2010; Van Kaer and Olivares-Villagómez, 2018).
Few reports focused on the effects of some antibiotics on the poultry immune response; therefore, more studies are needed to investigate the effects of those antibiotics on the immune system at the time of vaccination with Newcastle disease virus (NDV) Khalifeh et al. (2009). Tylvalosin (Acetylisovaleryltylosin tartrate) is an antibiotic used in poultry farms against Gram positive, some Gram-negative organisms and mycoplasma for treatment of respiratory and enteric infection. There are few publications describing its pharmacokinetics (Cerda et al., 2010). In poultry, tylvalosin is highly absorbed and distributed after oral administration in tissues especially respiratory tissues, bile, Liver, intestinal mucosa, spleen and Kidney. The main metabolite of tylvalosin is 3 acetyltylosin (3 AT) which has microbiological activity EMEA (2004). On the same line, tylvalosin acting as an antiviral drug as well as its metabolites play role on combating viral infection and afford satisfactory results Mockett et al. (2017).
This study highlights the effect of Lactobacillus acidophilus and tylvalosin on the immunity of ND vaccinated chickens and the influence of L. acidophilus and ND vaccine on kinetic and tissue residues of tylvalosin.
Material and Methods
Ethics
The study was approved by the Institutional Animal Ethics Committee of Veterinary Serum and Vaccine Research Institute (VSVRI). All procedures and the care of chicks were in accordance with the institutional guidelines for animal use in research.
Drugs
Avilosin® (tylvalosin tartrate 62.5% w/w) obtained from ECO Animal Health, London, UK), administrated orally to 3 days old chicks with 25 mg/kg b.w. for 3 consecutive days. Tylvalosin was given at first days of life and repeated at risk time as vaccine administration EMEA (2004) and Stipkovits et al. (2007).
Instruments and Chemical reagents for HPLC procedures
Agilent HPLC Series 1200 quaternary gradient pump, Series 1200 auto sampler, Series 1200 UV Vis detector, and Agilent Chemstation software (Hewlett-Packard, Les Ulis, France ). Acetonitrile, methanol and water for preparation of mobile phase were HPLC grade (Fisher). Ammonium acetate (Merck). Tylvalosin and 3-O- acetyltylosin standards were purchased from Toronto Research Chemicals (Trc), Canada.
Bacterial strain
Lactobacillus acidophilus strain isolate was kindly obtained from Food Hygiene department at Animal Health Research Institute, Dokki, Giza, Egypt. Chickens received 1x107 CFU/chicken according to Brisbin et al. (2011) for 4 consecutive days. Reactivation of lyophilized L. acidophilus strain was done according to Shokryazdan et al. (2017).
Specific pathogen free embryonated chicken eggs (SPF-ECE)
(SPF–ECE) were obtained from the Specific Pathogen Free Egg Project, Kom Oshim, El-Fayoum Governorate, Egypt. The eggs were incubated at 37°C and 80% humidity until inoculated at 9-11 days of age via allantoic sac route and used for virus propagation and virus titration.
Live NDV vaccine
Lyophilized live NDV (LaSota strain) vaccine used in this study was locally prepared in the VSVRI, Abbasia, Cairo. It was titrated in (SPF-ECE) before used and its infectivity titer was 109 EID50 /ml Liu et al. (2008). Sterility test for live NDV vaccine was done according to USP (2009).
Challenge virus
Very virulent NDV (vvNDV) field isolate (infectivity titer 106 EID50/ 1 ml) used for the potency (challenge) test. It was administrated intramuscularly at 14 days old. It was obtained from the poultry viral vaccine department, VSVRI, Abbasia, Cairo, Egypt (Sheble and Rida, 1976).
Animals
One hundred and ninety specific pathogenic free (SPF) chicks at 1 day old were kept for 2 days to relief the stress, fed balanced ration with drinking water ad-libtum. SPF chicks were obtained from the SPF egg production farm,
Table 1: Experimental design of bird’s groups
| Groups name | Birds no. | Groups supplementations | Dosing | Duration | Administration time | Administration route | |
| T | 35 | Tylvalosin. | 25mg/Kg |
Once (kinetic) 3 days (residue study) |
3 days old |
Repeated before challenge time at 14 days old. |
Orally |
| VT | 35 | Tylvalosin | 25mg/Kg |
Once (kinetic) 3 days (residue study) |
3 days old | ||
| Live NDV vaccine | 1ml/ chick | Once | 7 days old | ||||
| VTL | 35 | Tylvalosin | 25mg/Kg |
Once (kinetic) 3 consecutive days(residue study) |
3 days old | ||
| L. acidophillus |
1x107 CFU/bird |
4 days | 4 days old | ||||
| Live NDV vaccine | 1ml/ chick | Once | 7 days old | ||||
| VL | 25 | L. acidophillus |
1x107 CFU/bird |
4 days | 4 days old | ||
| Live NDV vaccine | 1ml/ chick | Once | 7 days old | ||||
| V | 25 | Live NDV vaccine | 1ml/ chick | Once | 7 days old. | ||
| L | 25 | L. acidophillus |
1x107 CFU/bird |
4 days | |||
| Control | 10 |
Kept as non-vaccinated non challenged (control –ve) group. |
|||||
Koum Osheim , El-Fayoum , Egypt. The chicks were divided into 7 groups and housed in batteries (160 cm length, 140 cm width and 50 cm depth) at research laboratory, VSVRI, Abbasia, Cairo , Egypt. Groups treated with tylvalosin (T, TVL and TV) each consisted of 35 broiler chickens (12 for kinetic study; 23 for residue study).
Experimental Design: as described in Table (1)
Challenge test (OIE, 2012):
All vaccinated groups (VT, VTL, VL, V) and unvaccinated (control, T, L) groups were challenged intramuscularly with 105 EID50 units of vvNDV (very virulent NDV) at 2 weeks post vaccination. Chicks were observed for 14 days and the protection degree was assessed according to the severity of clinical signs and the mortality associated with virus challenge.
Sample collection
For pharmacokinetic study; about 1 ml blood were taken from the wing or leg veins at 0.25, 0.5, 1, 2, 4, 6, 8, 10, 12- and 24-hours post tylvalosin administration. Blood samples were left to clot for 30 min, centrifuged at 3000 rpm/15 min and the obtained clear serum was transferred to eppendorff ‘s tubes and kept in the deep freeze (-20oC) till assayed.by HPLC assay.
For residue study; three chickens were slaughtered at 1st, 2nd, 3rd, 4th, 5th and 6th day after the last oral dose. Samples from lung, liver, kidney and muscle were taken from slaughtered chickens for determination of tylvalosin and its metabolite (3-O-acetyltylosin) residues by HPLC assay.
For studying the effect on the poultry immune response after ND vaccination in vaccinated groups with ND and control group, the serum samples for serology test were collected regularly; (weekly till 6 weeks post vaccination for detection of serum antibodies against NDV using Hemagglutination Inhibition test (HI) test according to (OIE, 2012) and at 1st, 3rd and 5th day post vaccination for determination of serum IL-8 using competitive inhibition ELISA system (CUSABIO TECHNOLOGY LLC, China) according to the manufacturer’s instructions. The color change was detected spectrophotometrically at a wavelength of 450 nm and the concentrations of the samples were interpolated on the standard curve (Zwaini, 2017).
Development of HPLC method and validation
Tylvalosin extraction from serum and tissues samples according to (Hurtaud-Pessel et al., 2011) with some modifications for determination of tylvalosin and its metabolite (3-acetyltylosin) as recommended by (EMA, 2004).
Two grams of tissues homogenate (0.5 ml for serum) were weighed in 15 ml centrifugal tube and 2 ml of acetonitrile (ACN) (0.5 ml for serum) were added. The samples were mixed for 10 min then centrifuged at 14,000 xg/5 min. The previous steps were repeated for one more time. The collected supernatants were collected and evaporated to dryness under nitrogen stream at 50°C. The remaining residue was dissolved in 0.5 ml of 0.2 M ammonium acetate and then purification using solid phase extraction (SPE) cartridge (C18, 500mg/6ml). The cartridge was conditioned by 2ml methanol followed by 2ml water, and then the sample was loaded and washed with 1 mL of 25% methanol in water and dried for 10 min. The sample was eluted by 0.5 ml of mobile phase then injected to HPLC system.
Chromatographic conditions
Agilent C18 column (4.6mm i.d, 250mm, 5µm particle size) at ambient temp., with 10 µl injection volume and flow rate 1 ml/min. Mobile phase (0.02 M ammonium acetate: methanol) (30:70 v: v). Peak areas in the sample chromatograms were quantitated by an external standard technique using solutions of tylvalosin and 3-O-acetyltylosin reference standards. The analytical method was fully validated according to EU requirements for tylvalosin and its metabolite [specificity, linearity and range, precision, recovery and accuracy, limit of detection (LOD) and quantification (LOQ) according to (USP, 2017).
Statistical analysis
The obtained results were subjected to Statistical analysis as all the parameters were analyzed by one- way ANOVA for comparison between groups using a complete randomized design (Kim, 2014). The differences between groups means were further compared by Duncan’s multiple range test. Differences with a P value below 0.05 were considered statistically significant. The pharmacokinetic parameters were analyzed by PK solver. An add-in program for Microsoft Excel, version 2, and other parameters were calculated according to (Zhang et al., 2010).
Results
Method Validation and Verification
Tylvalosin and its metabolite standards were prepared at concentrations of 0.02 – 10 µg/ml in Fig 1-a, and were prepared in the mobile phase with correlation coefficients (R2) 1 and 0.999, respectively. The inter-day precision was 0.05% and intraday precision was 0.9%. The recovery of Tylvalosin was ranged from 96.9-102.41% for serum and tissues. Accuracy was 99.3 ± 1.36. Robustness was evaluated by pooled RSD% which was less than 3.1%. LOD and LOQ were 0.01 and 0.03 µg/g, respectively. Specificity was evaluated through calibrated chromatogram of tylvalosin and its metabolite either in standard preparation or serum and tissues samples. They were specific at retention time 1.509 and 1.91min. for 3-AT and tylvalosin, respectively. Fig 1-b and Fig 1-c.
Pharmacokinetic Results
The oral kinetic Parameters of tylvalosin in all treated groups (the sum of 3-O-acetyltylosine and tylvalosin) were illustrated in Table (2) and Fig (2).
Tissue distribution results
Residues depletion of tylvalosin (sum of tylvalosin and its metabolite) after repeated oral dose of tylvalosin (25
mg/ Kg b.wt) for 3 consecutive days in all described groups were illustrated in Table (3).
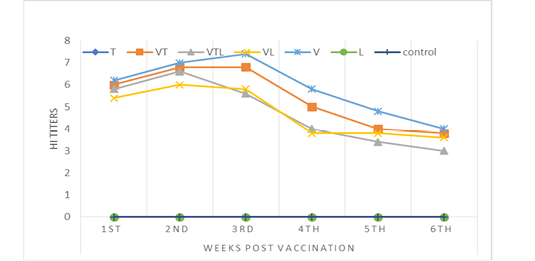
HI antibody titer
The antibody titer of NDV vaccine was measured by HI test for 6 weeks post vaccination. The obtained results were detailed in Table (4) and Fig (3). The data proved that there wasn’t any significance between V and VT groups through the experiment time. Mostly VL and VTL groups declared no significance between each other but with highly significant decrease than V and VT groups.
Table 2: Pharmacokinetic parameters of Tylvalosin after oral administration (25 mg/kg b.wt.) for 3 consecutive days in different treated groups (n=3)
| Parameters and Units | Gr. T | Gr. VT | Gr. VTL |
| Ka (1/h) |
0.44±0.02a |
0.46±0.1a |
0.45±0.1a |
| t1/2ka (H) |
1.6±0.1a |
1.5±0.4b |
1.5±0.1b |
|
t1/2 α (Alpha) (H) |
1.8± 0.01a |
1.6±0.001b |
1.6±0.03b |
| t1/2Beta (H) |
5.7±0.02c |
6.1±0.02b |
7.3±0.02 a |
| V/F (µg/ml) |
8.001±0.1c |
9.1±0.03a |
8.4±0.2b |
| CL/F (µg/ml/h) |
1.04±0.5a |
1.2±0.02b |
1.2±0.2b |
| T max (H) |
3.18±1.1c |
3.8±0.9a |
3.4±0.5b |
| C max (µg/ml) |
1.8±0.02a |
1.15±1.1c |
1.5±0.7b |
|
AUC 0-t (μg/ml*h) |
22.2±0.2a |
18.3±0.1c |
19.1±0.9b |
|
AUC 0-inf (μg/ml*h) |
24.001±0.3a |
20.01±0.1c |
21.5±1.1b |
| MRT (H) |
10.3±0.02b |
10.5±0.01b |
11.7±0.02a |
The data carry different small letter within the same row is considered significant at p<0.05
CL/F: Total body clearance of drug; V/F: Volume of distribution; T1/2α: distribution half-life time; T1/2β: Elimination half-life time; Cmax: maximum serum concentration; Tmax: time in which drug reached to maximum serum concentration; Ka: absorption rate constant; t1/2 Ka: half-life time of absorption; AUC0-t: Area under the curve from zero to time; AUC0-inf: Area under the curve from zero to infinite time.
Table 3: Tissue residues of TVN and its metabolite after oral administration (25mg/ Kg b.wt) of broiler chicken in different groups (n=3)
| Tissues | Groups |
Days post administration Concentrations (µg/g), Mean ± SD |
|||||
|
1st |
2nd |
3rd |
4th |
5th |
6th |
||
| Lung | T | 5.48±0.15 | 2.16±0.09 | 1.12±0.07 | 0.437±0.04 | 0.033±0.005 | ND |
| VT |
2.98±0.07a |
1.29±0.06a |
0.625±0.02a |
0.322±0.02a |
ND | ND | |
| VTL |
3.207±0.1ab |
1.497±0.02ab |
0.711±0.01ab |
0.282±0.02ab |
ND | ND | |
| Liver | T | 3.053±0.1 | 1.51±0.016 | 0.788±0.02 | 0.205±0.009 | ND | ND |
| VT |
1.765±0.11a |
0.94±0.04a |
0.436±0.02a |
ND | ND | ND | |
| VTL |
2.359±0.4ab |
1.022±0.01a |
0.552±0.02ab |
0.312±0.03a |
ND | ND | |
| Kidney | T | 1.094±0.01 | 0.419±0.02 | 0.142±0.01 | ND | ND | ND |
| VT |
0.677±0.04a |
0.351±0.05a |
ND | ND | ND | ND | |
| VTL |
0.792±0.02ab |
0.360±0.02a |
ND | ND | ND | ND | |
| Muscles | T | 0.809±0.012 | 0.289±0.03 | ND | ND | ND | ND |
| VT |
0.451±0.02a |
0.257±0.02a |
ND | ND | ND | ND | |
| VTL |
0.628±0.04ab |
0.236±0.03a |
ND | ND | ND |
ND |
|
a: Significant change at p<0.05 with respect to group T using ANOVA test.
b: Significant change at p<0.05 with respect to group VT using ANOVA test.
Table 4: The mean log2 of HI antibody titer for chicken vaccinated with NDV vaccines after single vaccination
| Group | Weeks post vaccination | |||||
|
1st |
2nd |
3rd |
4th |
5th |
6th |
|
| T |
0.0 ± 0.0c |
0.0 ± 0.0 d |
0.0 ± 0.0c |
0.0 ± 0.0d |
0.0 ± 0.5c |
0.0 d |
| VT |
6.0 ± 0.4a |
6.8 ± 0.4a |
6.8 ±1.3 a |
5.0 ± 1.5ab |
4.0 ± 1.3a |
3.8 ± 1.09a |
| VTL |
5.8 ± 0.7ab |
6.6 ± 1.1bc |
5.6 ± 0.5ab |
4.0 ± 0.7c |
3.4 ± 0.8ab |
3.0 ± 1.0 c |
| VL |
5.4 ± 1.1ab |
6.0 ± 1.5c |
5.8 ± 1.09ab |
3.8 ± 0.8 c |
3.8 ± 1.3ab |
3.6 ± 0.5bc |
| V |
6.2± 0.8a |
7.0 ± 0.0 a |
7.4 ± 0.7 a |
5.8 ± 0.5 a |
4.8 ± 1.6a |
4.0 ± 0.8a |
| L |
0.0 ± 0.0c |
0.0 ± 0.0 d |
0.0 ± 0.0c |
0.0 ± 0.0d |
0.0 ± 0.5c |
0.0 d |
| control |
0.0 ± 0.0c |
0.0 ± 0.0 d |
0.0 ± 0.0c |
0.0 ± 0.0d |
0.0 ± 0.5c |
0.0 d |
Means within the same column carrying different superscripts are significant at p<0.05

Figure 2: Mean plasma concentrations versus the time-course of sum of tylvalosin and its metabolite in serum of broilers after administration of tylvalosin 25 mg/kg b.wt in groups (T; TV &TVL) (Data represent mean ± SD values for 3 chickens).
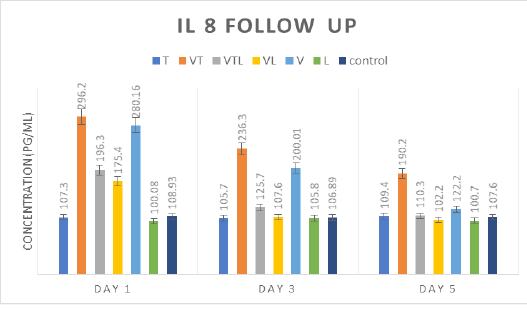
IL-8 follow up
Through the delivered results in Table (5) and Fig (4), IL8 secretion on 1st and 3rd days was statistically higher in VT and V groups than the other groups (T, VTL, VL, V and control). Also, there were significant IL 8 secretion increment as the follow order; VTL and VL > T> L and control groups. On 5th day, VT and V kept the significance higher level than the other groups which reported nearly normal levels as control group without any significance.
Table 5: IL 8 mean concentration for all tested groups
| Groups | Days | ||
|
1st |
3rd |
5th |
|
| T |
107.3±0.2bc |
105.7±0.3bc |
109.4±0.1c |
| VT |
296.2 ± 0.7a |
236.3±0.1a |
190.2±0.5a |
| VTL |
196.3 ± 0.5ab |
125.7±0.7ab |
110.3±0.2c |
| VL |
175.4 ± 0.1ab |
107.6±0.7ab |
102.2±0.3c |
| V |
280.16 ± 0.8a |
200.01±0.4a |
122.2±0.6b |
| L |
100.08±0.2bc |
105.8±0.3bc |
100.7±0.2c |
| control |
108.9 ± 0.3bc |
106.89±0.5bc |
107.6±0.1c |
Means within the same column carrying different superscripts are significant at p<0.05
Challenge test results
Challenge test results in Table 6 illustrated maximum protection rate (100%) in all vaccinated groups (VT, VTL, V) except VL group (93.3%). On the other hand, the non-vaccinated groups (T, L and control) recorded 66.6%, 40% and Zero, respectively.
Table 6: Protection percentage for vaccinated and non-vaccinated chicken groups two weeks post vaccination
|
Chicken Groups |
No. of Birds | Protected birds | Dead birds | Protection (%) |
| T | 15 | 11 | 4 | 66.6 |
| VT | 15 | 15 | 0 | 100 |
| VTL | 15 | 15 | 0 | 100 |
| VL | 15 | 14 | 1 | 93.3 |
| V | 15 | 15 | 0 | 100 |
| L | 15 | 6 | 9 | 40 |
| control | 15 | 0 | 15 | (0) |
Protection % was calculated according to the following equation:
Protection %=Life birds/Total No. of birds x 100
Discussion
Tylvalosin is one of the most common antimicrobial drugs against mycoplasma regardless its immunomodulatory effect on chickens. Many farmers utilize vaccines and antibiotics as the main control measures against viral and bacterial diseases, respectively, with no enough scientific information regarding the effect of these antibiotics on the immune response of chickens and so it is important to study the effects of different antibiotics on the immune system (Khalifeh et al., 2009). As the same, with increasing interest in using probiotics as substitutions to antibiotic growth promoters in animal production systems, it is very critical to understand the role of probiotic bacteria in modulating the host immune system (Khaksefidi and Ghoorchi, 2006) Although the immunomodulatory activities of probiotic bacteria are not fully studied, it has been demonstrated that some probiotics alter the adaptive immune response. Others have linked changes in the systemic immune response to the ability of probiotic to cause changes in T cell populations and functions leading to an increase in regulatory T cells (Lavasani et al., 2010) and (Mazmanian et al., 2005) or its role to enhance the ability of dendritic cells for antigen presentation (Tsai et al., 2008).
The present study was designed to examine the effect of probiotic (L. acidophilus) administered individually or in combination with antibiotic (tylvalosin), on the antibody immune responses of chickens vaccinated with live Newcastle vaccine. Besides, the influence of L. acidophilus and ND vaccine on kinetic and tissue residues of tylvalosin.
The distribution half life time (t0.5 α) of tylvalosin group (group T) was (1.8 ±0.01 h) which was higher than tylvalosin and vaccine group (Gr VT) (1.6 ±0.001 h) and tylvalosin, vaccine and lactobacillus group (group VTL) (1.6 ± 0.03 h). These results were in consistent with (Radi, 2016) and (Vermeulen, 2002) who recorded that lactobacillus flora inactivate macrolides activity and interferes the absorption of tylvalosin. This explains the prolongation of mean residence time (MRT) in Gr. VTL (11.7±0.02) than Gr. T (10.3±0.02). In consequence with this evidence Cmax and Tmax of group VTL were 1.5± 0.7 µg/ml and 3.4 ±0.5 h, respectively, However, Group T achieved the higher serum maximum concentration (Cmax) of (1.8± 0.02µg/ml) which was reached in Tmax (3.18 ±1.1 h) which similar to data recorded by (Elbadawy et al., 2019). Contrarily, group VT had got the lowest Cmax 1.15± 1.1 µg/ml at the longest time (Tmax 3.8 ±0.9 h) between the other groups. Also, its volume of distribution (V/F) value (9.1±0.03 µg/ml) was significantly higher than Gr. T (8.001±0.1µg/ml) and Gr VTL (8.4±0.2 µg/ml). These may be due to interferon interference blockage phenomena for NDV which explained by (Ginting et al., 2019) as NDV strongly stimulate both type I and II antiviral interferons (IFNs-α/-β and IFN-λ, respectively) signals to cell membrane bound Toll like receptors (TLR), while tylvalosin act as antiviral agent through rapidly entered and accumulated into white blood cells (neutrophils) of chicken specially endosomes and lysosomes. Tylvalosin concentrated in early stage or late-stage endosomes and raise pH which generally prevent propagation of the viruses (Mockett et al., 2017). So, interferon blockage mentioned above by NDV prevented the drug to be concentrated into the cells which explain the decreased blood parameters and tissue residues in Gr VT.
The observed pharmacokinetic results of this study declared that all groups had significant differences between each other. The mean maximum serum concentration of Gr T was reached after 2 hrs. of drug administration and absorption half-life (t0.5Ka) was significantly higher in Gr. T (1.6±0.1 h) than the other groups. The same was recorded by (Abo El Ela et al., 2015) and unlikely with (Salman, 2017). Significant changes in total body clearance of tylvalosin (CL/F), Elimination half-life (t0.5beta) and Area under curve (AUC0-t, AUC0-∞) were noticed. This might due to the increased production of adrenocorticotropic hormone (ACTH) by NDV (Lam et al., 20011). ACTH stimulated adrenal cortex to release corticosterone which augmented hemostatic mechanism and so more elimination time (Wang et al., 2015). Lactic acid bacteria (LAB) augmented the effect of vaccination. LAB reduced bile acids in bile (Deng et al., 2020) but tylvalosin mainly excreted through bile. Moreover, its interaction with bile acids strengthens its action (Glanzer et al., 2015).
Distribution of tylvalosin residues in tissues was found in the highest concentrations in the lung, liver and kidney in Gr T. On the other hand, in the all-tested groups (T, VT and VTL), tylvalosin muscles residues were at the lowest concentrations and disappeared after 2 days of drug administration in harmony with data reported by (EMA, 2004) and (Salman, 2017). Gr VT showed a significant decrease in tylvalosin level than the two other comparable groups (T and VTL) in the all examined tissues (lung, liver, kidney and muscles). According to the former results, it was observed that the vaccination decreases tylvalosin concentration levels inside poultry tissues and this may be due to the immunostimulant effect of the vaccine. Otherwise, the revealed data in Gr. VTL proved that lactobacillus flora decrease the absorption of tylvalosin and accordingly its distribution in tissues as (Salman, 2017). There was significant decrease in tissue residues in Gr. VTL and high significant decrease in Gr VT than Gr T. Also, tylvalosin was detected in lung with highest concentration than other tissues till 5th day after treatment cessation in Gr T, but in groups (VT and VTL) the drug detected till 4th day. Overall, Gr T and Gr TVL showed significant decrease in drug residues concentration. Besides, (EMA, 2004) proposed that the minimal inhibitory concentration (MIC) of tylvalosin in chicken for M. gallisepticum ranges from 0.007 to 0.25 μg/ml. This was a supportive point with our study as in all groups; tylvalosin residues in lung were kept above MIC till disappeared. This indicated that NDV vaccine and L. acidophilus didn’t change the efficacy of tylvalosin against any probably M. gallisepticum infection. Maximum residual Limit (MRL) of tylvalosin was 50µg/kg in muscle, kidney and liver tissues according to (EUROPEAN COMMISSION, 2010). In this study, residues concentration was above MRL till disappearance but QL of our validated HPLC method was 30µg/kg. So, the residues weren’t quantifiable under 30µg/kg and so below MRL. Therefore, it will be safe for human consumption.
The Antibodies against NDV detected by HI test revealed that the highest mean log2 serum antibody titer was in Group V (vaccinated by live NDV) between all examined groups (VT, VL, and VLT) and the peak of antibody titer was in 3rd week as (7.4 log2). On the other hand, the peak of antibody titer of group VL (vaccinated by ND vaccine and treated with L. acidophilus) became clear in 2nd week post vaccination as (6.0 log2) that was the lowest peak among other groups. Generally, the vaccinated groups that treated with L. acidophilus show the lowest level of antibodies titer (group VL and VLT) in comparing with other groups (V and VT). These results agreed with (Brisbin et al., 2011) who proved that L. salivarius and L. acidophilus both demonstrated weak immunity-enhancing effects. Also, L. acidophilus was down-regulated pro-inflammatory cytokines IL-8 and TNF-α in vivo based on animals’ experiments (Li et al. 2016; Moges et al. 2018). Contrarily, (Khalifeh et al., 2009) reported that antibody titer in chicks delivered probiotic with NDV vaccine was significantly higher 10 days post immunization than control.
Tylvalosin showed a slightly suppressor effect on chickens’ humoral immune response. The group VT (treated with Tylvalosin) showed immuno-inhibitory effect on the HI Ab titers when compared with group V (ND vaccine). The decrease in the HI Ab titers may be attributed to the suppressive effects of tylvalosin on lymphocyte (LiRuChun, 2008; Moges et al., 2018). Moreover, tylvalosin was reported to decrease the pro-inflammatory cytokines as IL-6, IL-8, IL-1β, PGE2, TNF alpha that have a role in mediating the humoral immune response (Zwaini, 2017). This result compatible with (F.I.A. El-Ela et al., 2016) who clarified that tylvalosin suppress both humoral and cellular immune response of chicks. Contrarily, depending on the triggering effect of NDV vaccine on humeral immunity with the hemostatic action of tylvalosin triggered by corticosterone may explain that no significant difference in IL-8 level between V and VT groups. Increasing hemostatic action stimulated inflammatory mediators as IL-8 ESMON (2005).
The potency test on chicks under test showed that vaccinated group treated with L. acidophilus only (group VL) showed the lowest protection% (93.3%) among all vaccinated groups (V, VT, and VLT) and this might because of the weak immunity-enhancing effects of L. acidophilus that clarified by (Brisbin et al., 2011). Similarly, the group treated with L. acidophilus showed lowest protection % (40%) when compared with group T treated with tylvalosin that showed protection% reached to (66.6%) and this might due to the hypothesis of tylvalosin action mechanism as antiviral drug (Mockett et al., 2017) The virus replication mechanism needs certain pH to accomplish this mission. Tylvalosin modulate pH inside endosomes and lysosomes after their internalization which leads to stop or inhibit viral replication.
The group V (ND vaccine) and vaccinated groups treated with Tylvalosin (VT and VLT) showed full protection % (100%) and these results close to the study conducted by (Khalifeh et al., 2009) who studied the effect of some antibiotics on the immune response, where they were demonstrated that these antibiotics clearly targeted the humoral immune response and resulted in a decrease in antibody production, although this decrease does not affect the protection of birds. Therefore, the use of tylvalosin at the same time during NDV vaccination can be encouraged without causing major downregulatory effects on antibody protection outcome from the vaccination.
Conclusion
We concluded that the exposure of chicks to tylvalosin within their early life had a synergistic action with NDV vaccine as it had no negative effect on the immunity to NDV vaccine which enhanced significantly tylvalosin pharmacokinetics and residues. In addition, tylvalosin acts as antiviral drug beside its antibiotic activity. Therefore, its administration could provide a notable protection with accidental NDV infection. L. acidophilus didn’t cause any significant amelioration. Through the reported findings, tylvalosin is the recommended antibiotic for use with NDV vaccine and L. acidophilus alone isn’t recommended for broilers as a probiotic.
Authors contribution
Mai A. Fadel: designed the experiment, performed HPLC method validation, analyzed the samples for pharmacokinetics and residues detection on HPLC and analyzed the data statistically. Maha S. Abd El-Hafeez: participated in pharmacokinetic and tissues residues analysis on HPLC. Mahmoud Mohamed Abd El-Moneam: participated in execution of the experiment, performed HI test, challenge test and IL-8 assessment. All authors wrote and revised the full manuscript.
Acknowledgments
None to declare.
Conflict of interest
No conflict of interest was declared.
References