Advances in Animal and Veterinary Sciences
Research Article
The Antimicrobial Potential of Selenium Nanoparticles Singly and in Combination with Cinnamon Oil Against Fungal and Bacterial Causes of Diarrhea in Buffaloes
Atef A. Hassan*, Mareim H. Yousif, Hanaa M. M. Abd-Elkhaliq, Ahlam K. A. Wahba, Ahmed M.a. El-Hamaky
Microbiology-Animal Health Research Institute (AHRI), Agriculture Research Center(ARC),Dokki, Giza Egypt.
Abstract | The antimicrobial potential of selenium nanoparticle (SeNPs) singly or in combination with cinnamon oil were evaluated against causes of diarrhea in buffaloes. The pathogens of E. coli and C. albicans were recovered from animal feeds, drinking water and feces of diarrheic animals at the top of all other iso-lates. The green synthesized selenium nanoparticles and cinnamon oil were had significant antimicrobial potential than traditional antibiotics against E. coli and C. albicans. The inhibitory concentrations of Se-NPs against C. albicans and E. coli were (0.4 and 0.3 mg/ml) and for Cinnamon oil were (0.5 %), respec-tively. The combination of SeNPs and Cinnamon oil completely inhibited the growth of C. albicans and E. coli sp. at (0.2 mg Se NPs / 0.2 % Cinnamon oil). It is concluded that the synergistic activity of metals NPs Se NPs / cinnamon oil were urgently required to overcome the microbial resistance against the traditional antibiotics and decrease the concentrations used of nanoparticles to avoid its toxicity for animals.
Keywords | Selenium nanoparticles, Synergy, Nano-emulsion, Cinnamon oil, Antimicrobial, Nanotechnology
Received | February 06, 2021; Accepted | March 17, 2021; Published | July 15, 2021
*Correspondence | Atef Hassan, Microbiology-Animal Health Research Institute (AHRI), Agriculture Research Center(ARC),Dokki, Giza Egypt; Email: atefhassan2000@yahoo.com
Citation | Hassan A, Yousif MH, Abd-Elkhaliq HMM, Wahba AKA, El-Hamaky AMA (2021). The antimicrobial potential of selenium nanoparticles singly and in combination with cinnamon oil against fungal and bacterial causes of diarrhea in buffaloes. Adv. Anim. Vet. Sci. 9(8): 1238-1248.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.8.1238.1248
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Hassan et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Nowadays, there is a huge worldwide interest in applications of nanotechnology in different fields of biomedicine related to human and animal health (Hassanen et al., 2019; Khalaf et al., 2019). Where, the animal health and their production represent the major role in food security for human consumption and of huge economic importance in developing countries (Patel et al., 2010; Hassan et al., 2020b). The microbial infections resulted from opportunistic bacteria and fungi have been common especially in human and animals which being affected by special conditions likes’ immune weakness. However, the fungal infections particularly by C. albicans and mycotoxigenic mold represent the widest spread causes of mycosis diseases of man and animals (Refai et al., 2014; Hassan et al., 2015, 2016, 2019a, 2020c). Moreover, several fungal and bacterial diseases adversely affect animal as mastitis, diarrhea and respiratory tract infections that resulted in decrease in their production and industry. The most important effects are related to economic losses due to decrease in milk yield (McDowell et al., 1995), diminished meat production due to diarrhea (Fagiolo et al., 2005) and respiratory disorders which are stress factors resulted in a bad production of animal (Quinn et al., 2002). The main recovered causes of animal diarrhea were Staphylococcus sp. Streptococcus sp., E. coli, C. albicans, Aspergillus sp. and Penicillium sp. (Yuan et al., 2012; Hassan et al., 2019a). However, in cases of calve diarrhea, enterotoxigenic E.coli (ETEC) are predominantly isolated which are producing toxin, Salmonellae sp. and Y. enteroclotica (Milnes et al., 2008). While, in water buffalo, S. typhimurium can induce a variety of clinical syndromes with different pathological lesions (Fagiolo et al., 2005). Some mold as members of Aspergillus sp., Penicillium sp. and Fusarium sp. caused diseases disorder in buffalo (Hassan et al., 2019a, 2020b) and aflatoxicosis in cattle (Hassan et al., 2016). The problems in control of these microbial infections are attributed to drug resistance, which occurred because of prolonged wrong use of antibiotics (Williams, 2000; Jeykumar et al., 2013). Hence, the microbial pathogens maintain multidrug resistant genes that can be transferred to other pathogens (Goffeau, 2008; Daka and Yihdego, 2012). Therefore, novel antimicrobial agents are needed to overcome resistance to traditional antibiotics (Nabawy et al., 2014; Singh et al., 2018).
Recently, nanotechnology enable the production of effective antimicrobial agents from Nano sized materials particularly metals (Tran and Webster, 2011; Hassan et al., 2020 a, b). In addition, several studies confirmed the antioxidant, antibacterial, and antifungal activities of several metal and metal oxide nanoparticles. Examples for these nanoparticles are zinc oxide nanoparticles (ZnO-NPs) (Hassan et al., 2020a), copper nanoparticles (CuO-NPs) (Hassan et al., 2017), silver nanoparticles (Ag-NPs) (Fouda et al., 2019), and gold nanoparticles (GNPs) (Hassanen et al., 2020). Metals particularly selenium is the fundamental component or a micronutrient that used in disease treatment and could neutralize malignancy and decrease disease frequencies (Zonaro et al., 2015; Geoffrion et al. (2020). The selenium nanoparticles can be produced by seed of Mucuna pruriens gave NPs of nearly (100–120 nm) and had IC50 (60 μg/mL) for inhibition the cell viability at 48 h. (Menon and Shanmugam, 2019). They detected that the preparation of SeNPs by green methods are cost-effective and environmental friendly and can be utilized further for future biomedical applications. This micronutrient is the primary part of glutathione peroxidase (GSH-Px), a cancer-inhibiting catalyst, which is associated with the protection of cells from oxidative stress (Ramamurthy et al., 2013; Kumari et al., 2018).The SeNPs reported to be more efficient than bulk selenium in activating selenoenzymes and have reduced toxicity (Mary et al., 2016; Jay and Shafkat, 2018). There are other several uses of SeNPs as antioxidant, anticancer and antimicrobial agents and protection from heart diseases (Ramamurthy et al., 2013, Nazıroğlu et al., 2017; Zhao et al., 2018). Recently, Meena et al. (2018) reported that the significant advantages of Nano-emulsions of oils are the simplicity, inexpensiveness, stability, versatility and the solubility of lipophilic substances that will protect them from degradation. Furthermore, the supplementation of oil with nanomaterials were successfully used in veterinary medicine as drug delivery agent and antimicrobial agents (Hassan et al., 2020a). Similarly, Abd-Elsalam and Khokhlov 2015, Hassan et al., 2020b) detected the antimicrobial potential of eugenol-Zinc Nano-emulsions against Fusarium sp. and E. coli. In addition, the contact of oil Nano-drops to the microbial membranes cause its adhesion, destruction and final death of pathogens (Meena et al., 2018). Therefore, the objectives of present article were to detect the prevalence of fungal and bacterial causes of diarrhea in buffaloes. The most prevalent microbial agents that recovered from the present study were used for evaluation the effects of SeNPs singly and in-combination with cinnamon oil in inhibition their activities. The minimum inhibitory concentrations were measured during all tests in comparison with traditional antibiotics. Moreover, the activities mechanisms of SeNPs and oils and their benefits were fully discussed.
MATERIALS AND METHODS
Samples
Ninety samples (30 of each of animal feeds, water, and feces) were obtained from a private animal farm suffering from diarrhea at Giza governorates aseptically in sterile McCartney bottles. The samples were transferred, as soon as possible, to the laboratory and kept in fridge until examination.
Commercial antibacterial, antifungals and other chemicals
A known commercial antibacterial, antifungal and reagents were purchased from Sigma Chemical Company (USA).
Selenium nanoparticles and cinnamon oil
The used SeNPs were synthesized by green method and were characterized by the laboratory of ALDRIK Sigma chemical company, USA. It was in amorphous powder form with 60 nm particle size. While cinnamon oil was purchased in crud form from Al Gomhorya chemical company, Egypt.
Bacteriological and serological examination
Samples were cultured onto MacConkey agar medium for 24 hrs. at 37oC, then a peptone water cultures were prepared from appeared colonies to inoculate biochemical tests (Quinn et al., 2002). While, serological identification for E. coli and Salmonella sp. was undertaken according to (Edwards and Ewing 1972; Neville and Bryant, 1986).
Mycological examination
The samples were prepared and examined for isolation of fungi as method as Refai et al. (2012). The samples were inoculated into Petri-dish plates contained Sabouraud’s dextrose agar (SDA) and incubated 3-5 days at 25-28oC and identification of appeared mold and yeast colonies were identified according to (Pitt and Hocking , 2009; Refai and Hassan, 2013).
Green synthesis and characterization of selenium nanoparticles (Inregole et al., 2010).
One 100 ml (10-1 M) sodium selenosulphate was treated with 10 ml 4% glucose solution and mixture was refluxed. The color of the solution changes from colorless to yellow after refluxing immediately and become orange after 30 minutes. The orange color solutions remained stable for months. The prepared nanoparticles were characterized via UV-visible spectra of each solution were measured in a SHIMADZU UV-1800 double beam digital spectrophotometer. XRD patterns were obtained on a Philips X’pert MPD X-ray diffractometer using Cu Kα (1.54059 Å) radiation with the X-ray generator operating at 45 kV and 40 mA. TEM images were obtained on JEOL 2010 microscopes. The TEM sample was prepared by dropping a sample suspension in ethanol on a Cu grid coated with a carbon film.
Measurement of MIC of prepared SeNPs and cinnamon oil against C. albicans and E. coli that isolated from diarrhea in buffaloes (CLSI 2008):
Statistical Analysis
The obtained data were computerized and analyzed for significance. Calculation of standard error and variance was according to SPSS 14, (2006).
RESULTS AND DISCUSSION
A total of ninety samples from animal feeds, drinking water and animal feces (30 of each) were examined for isolation and identification of bacterial and fungal pathogens of diarrhea in buffalo. The tabulated results in (Table 1) illustrated that the incidence rates of bacterial species were (50 %, 56.7 % and 83.3 %) for E. coli in animal feeds, drinking water and feces, respectively. While, it were (23.3 %, 16.7 % and 33.3 %) for S. typhi in samples, respectively. Meanwhile, E. coli and S. typhi were recovered from (63.3% and 24.4%) of total samples. However, isolates of E. coli, Salmonellae sp. and Y. enteroclotica were recovered from cases of calve diarrhea (Milnes et al., 2008). While, in water buffalo, S. typhimurium can induce a variety of clinical syndromes with different pathological lesions (Fagiolo et al., 2005). Another study reported that S. typhi causing bacterial gastro enteritis and some Salmonella sp. have the multidrug-resistant (Yan et al., 2004, Scallan et al., 2011). On the other hand, the environmental pollution by fungi affect upon the growth rate and health of human and animals and cause several diseases as thrush, candidiasis, aspergillosis, dermatophytosis and mastitis, aneamia, carcinogenic, tremor-genic, hemorrhagic, pulmonary edema, immunosuppressive and hormonal effects (Hassan et al., 2016; 2019a; b, 2020b; Asfour et al., 2009). Currently, as observed in (Table 2), the incidence rates of molds and yeast species in animal feeds, drinking water and feces were (83.3 % , 56.7 % and 30 %), for A. flavus, (30 % , 83.3 % and 33.3 %) for A. ocraceus and (80 %,80 % and 86.7 %) for C. albicans, respectively. C. albicans were recovered from (68%, 40% and 16%) of diseased cases in buffaloes with mastitis, diarrhea and respiratory disorders, respectively Hassan et al. (2014). Similar results were obtained by (Hassan et al., 2016, 2017, 2019a; 2020b) who recovered these fungi from cases of diarrhea and respiratory affections of buffaloes. In the present study, the most prevalent bacterial isolates in cases of diar
Table 1: Prevalence rates of bacterial species recovered from the examined samples
| Bacterial isolates | Types of samples (30 for each) | |||||||
| Animals feeds (30) | Drinking water (30) | Feces (30) | Total (90) | |||||
| No | % | No | % | No | % | No. | % | |
| E. Coli | 15 | 50 | 17 | 56.7 | 25 | 83.3 | 57 |
63.3 |
| S. typhi | 7 | 23.3 | 5 | 16.7 | 10 | 33.3 | 22 | 24.4 |
% calculated according to the number of samples examined (30)
Table 2: Prevalence rates of molds and yeast species isolated from the examined samples
| Molds and yeast species | Types of samples (30 for each) | |||||||
| Animals feeds | Drinking water | Feces | Total (90) | |||||
| No | % | No | % | No | % | No | % | |
| A.flavus | 25 | 83.3 | 17 | 56.7 | 9 | 30 | 51 | 56.6 |
| A.ochraceus | 9 | 30 | 25 | 83.3 | 10 | 33.3 | 44 | 48.8 |
| C .albicans | 24 | 80 | 27 | 80 | 26 | 86.7 | 77 | 85.5 |
% calculated according to the number of samples examined (30)
rhea was E. coli that recovered from (63.3%) and fungi of C. albicans from (85.5%) of all examined samples.
Moreover, the mixed infection by E .coli with C. albicans in diarrheic buffaloes was investigated. The results revealed that out of 30 examined of feces 10 were showed mixed infection and 5 out of 30 feed samples had mixed infection. But, no mixed infection by E .coli with C. albicans was detected in water samples (Table 3). Okela, (2010) and Blanchard P.C. (2012) who detected mixed infection of E.coli and yeast in cases of bovine Diarrhea reported similar findings.
Herein, Susceptibility of E. coli to commercial antimicrobial agents were illustrated in (Table 4) which showed that E. coli isolates were resistant for each of (Ampicillin, Kanamycin, Tetracycline, Trimethoprim sulfate) in a percentages of (100 %, 80 %,95 % and 100%), respectively. While the isolates were sensitive for Amikacin, Colestin, Ofloxacin (80%, 90 %, 100 %), respectively. Currently, the results in (Table 5) detected the sensitivity to commercial antifungals against C. albicans which was resistant for (Fluconazole) at the rate of (100 %). While, it was sensitive for (Itraconazole and Nystatin) (100 % for each).
Table 3: Prevalence of mixed infection with E .coli & C. albicans recovered from diarrhoeic buffaloes
|
Types of samples (30 for each) |
Prevalence of E .coli & C. albicans |
|
| No. | % | |
| Animals feeds (30) | 5 | 16.7% |
|
Drinking water (30) |
0 | 0% |
| Feces (30) | 10 |
33.3% |
Since microbes have progressively eroded the effectiveness of previously successful antibiotics by developing resistance, the emergence of resistant and more virulent strains
Table 4: Antibiotic sensitivity test of representative E.coli isolated from diarrhoeic buffaloes
| Antibacterial agents | Bacterial isolates | |||
|
E .coli (20) |
||||
| R | S | |||
| No | % | No | % | |
| Ofloxacin (10 µg) | 0 | 0 | 20 | 100 |
| Amikacin (10µg) | 4 | 20 | 16 | 80 |
| Colestin (100 µg) | 2 | 10 | 18 | 90 |
| Ampicillin (10 µg) | 20 | 100 | 0 | 0 |
| Kanamycin (10 µg) | 16 | 80 | 4 | 20 |
| Tetracyclin (30 µg) | 19 | 95 | 1 | 5 |
| Trimethoprim (25 µg) | 20 | 100 | 0 | 0 |
No = Number % = Percent . R = Resistant S = Senstive.
of bacteria and fungi has outpaced the development of new antibiotics. Therefore, there is an inevitable and urgent medical need for antibiotics with novel antimicrobial mechanisms (Whitesides, 2003). Several recent studies revealed that the metals nanomaterials present in different forms as ZnNPs, AgNPs, Sins, CuNPs, CS-CuNPs, core/shell (CS) NPs; polymer-coated NPs and others have significat antimicrobial potential (Hassan et al., 2019a, 2020a).They have prominent biomedical activity than their bulk material (El-Sayed and Kamel, 2020). It is being applied not only in the treatment and the prophylaxis of infectious diseases but also used as diagnostics tools of infections (Hassan et al., 2019a; 2020a). Whereas, Hassanen and Ragab (2021) evaluated the antibacterial effect of low doses (5 mg/kg bwt) nanoparticles of chitosan (Ch-NPs), silver (Ag-NPs), and chitosan-silver nanocomposites (Ch-Ag NCs) against experimentally chronic infection induced by methicillin-resistant S. aureus in rats. They resulted that mixing between chitosan and silver nanoparticles in one
Table 5: Antibiotic sensitivity test of representative C. albicans isolated from diarrhoeic buffaloes
|
Fungal Isolates (Numbers)
|
Antifungal Agent | |||||||||
|
Fluconazole (10 g) |
Voriconazole (1g) |
Itraconazole (10g) |
Nystatin (100g) |
AmphotericinB (100μg) |
||||||
| R | S | R | S | R | S | R | S | R | S | |
|
C. albicans (20) % |
20 100% |
0 0 |
12 60% |
8 40% |
0 0 |
20 100% |
0 0 |
20 100% |
10 50% |
10 50% |
S = Sensitive R = Resistant
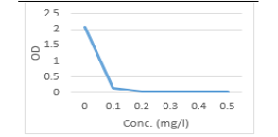
Table 6: Optical density and Transmittance of treated C. albicans and E. coli by Se NPs
.
| Concentration of Se NPs (mg/l) | C. albicans | E.coli | ||
| OD | T% | OD | T% | |
| 0.0 | 2.06 | 0.87 | 2.06 | 0.87 |
| 0.1 | 0.15 | 10.1% | 0.20 | 63.1 |
| 0.2 | 0.05 | 70.8% | 0.03 | 93.3 |
| 0.3 | 0.02 | 95.5% | 0.0 | 100 |
| 0.4 | 0.00 | 100% | 0.0 | 100 |
| 0.5 | 0.00 | 100% | 0.0 | 100 |
OD = Optical Density . T = transmittance
nanocomposite (Ch-Ag NCs) had a noticeable effect on bacterial count as well as the MIC value of this conjugate in vitro. In addition, they can be added in drinking water of broiler as in use of Gold NPs (0.5 mg/ kg of b.w.) which increased the growth performance and immune defense of broilers (Hassanen et al., 2020).
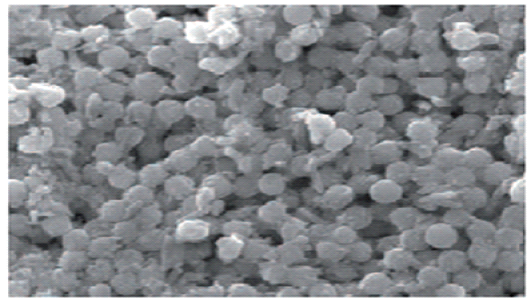
Herein, the used SeNPs was synthesized by green method to form glucose stabilized SeNPs from an aqueous sodium selenosulphate precursor under ambient conditions and the characterized NPs have amorphous powder form and the particles size was (60 nm) detected using TEM (Figure 1). The formation of selenium nanoparticles in presence of glucose is primarily authenticated from UV-Vis spectrophotometry shown in (Figure 2). They are safe methods and environmentally friendly and available for large-scale production. The organisms may cause changes in the toxic metals by decreasing their toxic effects (Inregole et al., 2010).
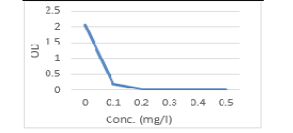
In the present study, SeNPs was evaluated for antimicrobial activities against the most predominantly isolated bacteria E. coli and fungi C. albicans from buffalo’s feed, drinking water and feces. The tabulated results in (Table, 6, Figure 3, 4), illustrated that the antimicrobial potential of Se-NPs against C. albicans, E. coli was concentration dependent. When the concentrations of SeNPs increased up to (0.5 mg/l), the OD of treated spore suspension were decreased until reach zero and T% increased to 100%. The inhibitory concentration of SeNPs against C. albicans was (0.4 mg/l) and it was (0.3 mg/l) for E.coli sp. Khiralla and El-Deeb (2015), detected the antimicrobial potential of Se-NPs against some bacterial food borne pathogens included E. coli, and S. Typhimurium and S. Enteritidis and MIC90 was (25 μg/ml for all). While, when the concentration of SeNPs reached (75 μg /ml), a complete inhibition of bacterial cells
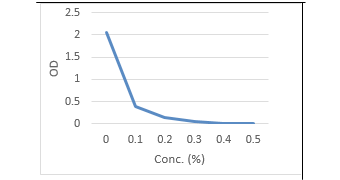
Table 7: Optical density and transmittance of treated C. albicans and E. coli by cinnamon oil.
| Concentration of cinnamon oil | C. albicans | E.coli | ||
| OD | T% | OD | T% | |
|
0.0 |
2.06 | 0.87 | 2.10 | 0.79 |
| 0.1% | 0.40 | 3.98 | 0.37 | 42.6 |
| 0.2% | 0.150 | 10.8 | 0.22 | 60.2 |
| 0.3% | 0.06 | 87.1 | 0.04 | 91.2 |
| 0.4% | 0.02 | 95.5 | 0.02 | 95.5 |
| 0.5% | 0.0 | 100 | 0.0 |
100 |
OD = Optical Density . T = Transmittance
Table 8: Optical density and transmittance of treated C. albicans and E. coli by Combination of Se NPs / cinnamon oil
| Concentration of SeNPs/CO | C. albicans | E. coli | ||
| OD | T% | OD | T% | |
| 0.0 | 2.06 | 0.87 | 2.10 | 0.79 |
| 0.1mg Se NPs/0.1% CO | 0.40 | 3.98 | 0.37 | 42.6 |
| 0.1 mg Se NPs/ 0.2% CO | 0.150 | 10.8 | 0.22 | 60.2 |
| 0.2 mg Se NPs/ 0.1% CO | 0.06 | 87.1 | 0.04 | 91.2 |
| 0.2 mg Se NPs/0.2% CO | 0.00 | 100 | 0.00 | 100 |
OD = Optical Density . T = Transmittance
growth occurred. Jay and Shafkat (2018) biosynthesized SeNPs and detected their anti-microbial activity against S. aureus and B. subtilis. They found that the MIC detected at (25 μl) and the highest zone of inhibition observed in S. aureus (32mm) and lower in B. subtilis (28mm) at concentration of 100μl SeNPs. While, Menon et al. (2020) detected antibacterial activity of SeNPs for wide range of bacterial strains. However, their antimicrobial activity have been detected against pathogenic bacteria, fungi and yeasts (Shahverdi et al., 2010, Hariharan et al., 2012, Beheshti et al., 2013; Hassan et al., 2019a b).
Regarding, the antimicrobial potentials of essential oil, they have antibacterial and antifungal activities (Vitoratos et al., 2013). They are able to control microorganisms related to skin and food spoilage, including Gram-negative and Gram-positive bacteria. Clove, cinnamon, mandarin, lime, and basil oils are the best examples that are commonly used as natural antibacterial and antifungal agents, attracting the growing interest of scientists for use as food preservatives (Ghosh et al., 2013).
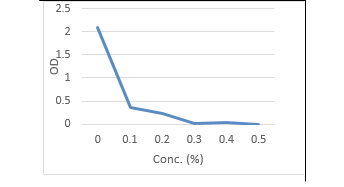
Currently, the present results of the antimicrobial potential of Cinnamon oil against C. albicans and E. coli sp. (Table, 7 and Figure 5, 6), yielded that the Optical density and transmittance were also concentration dependent. When the concentration of Cinnamon oil increased up to (0.5 %), the OD of treated spore suspension was decreased till reach 100 % T. The inhibitory concentration of Cinnamon oil that inhibited the growth of C. albicans and E. coli sp. was 0.5 % .Similar findings were detected by Eugénia et al. (2009) as he detected the significant fungicidal effect of eugenol, against Candida, Aspergillus and dermatophytes including fluconazole-resistant strains. In other study, Wahba and Abd-khaliq, (2013) recorded that the clove oil in a pure state has a bactericidal and fungicidal effect where it inhibit growth of bacteria such as St. aureus, St. lentus , dermatophytes as Microsporum canis, Trichophyton mentagrophyte and T. verrucosum at concentrations of (25, 20, 15, 10, 5 µl), respectively.
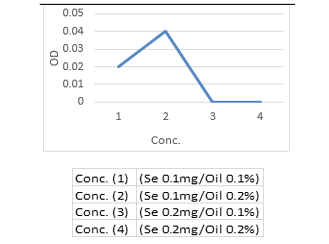
Moreover, herein the antimicrobial potential of synergistic activity of SeNPs with Cinnamon oil was evaluated. The findings in (Table, 8 and Figure 7, 8) indicated that the required concentrations for growth inhibition of C. albicans and E. coli sp. in combination of Se NPs and Cinnamon oil were at rates of (0.2 mg Se NPs / 0.2 % Cinnamon oil) which was lower than if each used separately (Table, 6 -8 and Fig. 1-6). The significance for using low concentrations of Se NPs by conjugation with cinnamon oil was to ensure their nontoxicity for human and animal cells.
Hassan et al. (2017, 2019 b and 2020b), detected the more antimicrobial potentials of conjugation of ZnNPs with cinnamon oil or ozone than their single activities against bacteria and fungi. In addition, the essential oil additives loaded into mesoporous silica nanoparticles (MSNs) have the ability to suppress the growth of A. niger (Bernardos et al., 2015). The combination of Nano-emulsions and matrix of certain nanostructures such as lipids and polysaccharides were more effective in inactivation of E. coli than the traditional and classical emulsions and used lower doses (Salvia-Trujillo et al., 2017).
On the other hand, there are several mechanisms of antimicrobial activity of NPs included contact of NPs and penetration of the cell walls, destroying a microbial cell generating ROS release of metals ions and caused oxidative stress (Rudramurthy et al., 2016). The release of metallic ions resulted in depolarization of cell membranes, lipid peroxidation, protein oxidation, and DNA damage (Huang et al., 2020). Based on oxidative stress, Chang et al. (2012) found that NPs may enter the microbial cells via endocytosis process this related to induction ROS and the ions released by the nanostructures. While, Jay and Shafkat (2018); Hassan, et al. (2019b) reported that Se-NPs caused destruction of cell wall, leakage of cytoplasm contents and loss of treated fungal and bacterial cell functions as detected when they subjected to SEM. Zhao et al. (2018), found that a high stress due to accumulation of SeNPs on surface of cells stimulated the production of ROS which help in inhibition of bacterial cells. In general, the antimicrobial effect of nanoparticles occurs by two ways (Moraru et al., 2003). The first is the formation of H2O2 on the surface of NPs due to the possible formation of hydrogen bond between hydroxyl group of cellulose molecules of fungi and bacteria. The synergistic and combination therapy of metals NPs as SeNPs with oils was urgently required to decrease the used concentration of nanoparticles, overcome the microbial resistant to traditional antibiotics, and resulted in more efficient antimicrobial activity of metal nanoparticles for the treatment of human and animal diseases.
Moreover, there will be several benefits of metallic nanomaterials to be used in improvement the biomedical applications. Although, data related to their harmful effects are not sufficient and special attention is required for known their toxicity risk before to biomedical applications. Hence, several toxicological studies are needed before nanotechnology applications in biomedicine and animal health.
CONCLUSION
The buffaloes’ diarrhea results in significant losses in animal health and causes important burdens to the country’s economy regarding to meat, milk, wool and leather industries. The frequent testing program of the animal feeds and other environmental factors for fungal and bacterial contamination is a critical demand.The metals nanomaterials are used as antimicrobial agents beside to other benefits strategies as diseases detection, diagnosis and therapy, additives to food, feeds and their products, and finally food safety. Our results detected that Se-NPs and cinnamon oil administration have significant antimicrobial potential against fungal and bacterial causes of diarrhea and their combination showed the requirement of lower concentrations from both to obtain the antimicrobial effects than their single form. Therefore, the synergistic therapies are needed to reduce the used doses of nanoparticles and hence overcome its toxicity and more efficient antimicrobial activities. Furthermore, the production of the production of SeNPs. Using glucose as a reducing agents and stabilizer avoid the aggregations of particles under ambient condition which can be used in large scale production and are safe method and environmentally friendly. The mechanisms of their activity are due to its penetration of cells membrane, damage of cytoplasmic contents, loss of function and cell kill. Hence, advanced and further investigations are required for direct treatment of farm animals by SeNPs in combination with other safe herbs and biological compound to avoid toxic effects of nanomaterials, which may result from misusing doses of nanoparticles. Moreover, the effects of green synthesized nanomaterial have long-term use on health need to concern and toxicity in surrounding environment and must be resolved in future. Therefore, the toxicity risk of nanomaterials must be determined before applications of green synthesized metallic nanomaterials in biomedicine for safe human and livestock health and their activities and production.
CONFLICT OF INTEREST
Authors declare that there is conflict of interest.
AUTHoRS’ CONTRIBUTION
All authors contributed equally.
REFERENCES