Advances in Animal and Veterinary Sciences
Research Article
Advances in Animal and Veterinary Sciences 2 (2): 106 – 110.Clinical Appraisal of 48 Cases of Obstructive Urolithiasis in Buffalo Calves Treated with Tube Cystostomy and Urethrotomy
Ram Bilash Kushwaha1*, Amarpal2, Hari Prasad Aithal2, Prakash Kinjavdekar2, Abhijit Motiram Pawde2
- Division of Teaching Veterinary Clinical Complex, Faculty of Veterinary Sciences & Animal Husbandry, Sher-e-Kashmir University of Agricultural Sciences and Technology–Jammu 181102
- Division of Veterinary Surgery, Indian Veterinary Research Institute, Izatnagar, Bareilly, 243 122 (UP), India
*Corresponding author: kushwaharb@rediffmail.com
ARTICLE CITATION:
Kushwaha RB, Amarpal, Aithal HP, Kinjavdekar P, Pawde AM (2014). Clinical appraisal of 48 cases of obstructive urolithiasis in buffalo calves treated with tube cystostomy and urethrotomy. Adv. Anim. Vet. Sci. 2 (2): 106 – 110.
Received: 2013–12–09, Revised: 2013–12–24, Accepted: 2013–12–25
The electronic version of this article is the complete one and can be found online at
(http://dx.doi.org/10.14737/journal.aavs/2014/2.2.106.110)
which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
Abstract
A clinical appraisal of forty eight buffalo calves suffering from obstructive urolithiasis and treated with tube cystostomy, urethrotomy and tube cystostomy plus urethrotomy (16 each) was made. All the affected calves were male and average age and duration of illness was 4.86 months and 4.20 days, respectively. Urinary bladder was intact in 19 calves and ruptured in remaining 29 calves. The common clinical signs with intact urinary bladder were anuria, inappetance to anorexia, sunken eyes, engorged urethra, anal sphincter movement, to and fro movement of the penis, frequent attempt to urinate etc. In the cases of ruptured urinary bladder, bilateral distension of abdomen was the common clinical sign. Rupture of urinary bladder on dorsal surface was more than on ventral surface. Appearance of bladder was mostly rough, inflamed and necrosed in ruptured and subserous ruptured cases whereas smooth and bluish in intact urinary bladder. Adhesion of bladder was seen with omentum, mesentery and/or peritoneum. Post–scrotal was the most common urethrotomy site and calculi were mostly retrieved from distal part of sigmoid flexure. In most of the cases, calculi were removed in single incision and catheterization was difficult to very difficult in those cases where multiple incisions were made in the urethra. Immediate flow of urine through urethral catheter was not seen in most of the cases; however, it was noticed through Foley’s catheter in all the cases. Duration of surgery was minimal in tube cystostomy cases.
Introduction
Urolithiasis is the formation of urolith(s), which may lodge anywhere in the urinary system but most frequently at the distal end of sigmoid flexure in ruminants and causes obstruction to urine flow (Radostits et al., 2000; Kushwaha et al., 2011). The clinical signs and physiological parameters of urolithiasis may vary with the degree of urethral obstruction, its duration, age and sex of the animals, and status of urinary bladder and urethra. Similarly, intra–operative observations may vary according to condition of the animals and surgical procedure adopted. Though earlier authors have several observations on urolithiasis (Lavania et al., 1973; Gera and Nigam, 1979; Singh and Singh, 1990; Sharma and Singh, 2001; Kushwaha et al., 2011), detailed studies on different types of clinical, physiological and intra–operative are limited particularly in buffalo calves. The present paper describes the clinical and intra–operative observations made on buffalo calves suffering from complete urethral obstruction.
MATERIALS AND METHODS
Forty eight buffalo calves, brought to the Referral Veterinary Polyclinic, Izatnagar, Bareilly, UP, India with the history of the urethral obstruction were included in the study. Sixteen animals each were treated with tube cystostomy (Van Metre, 2004; Singh, 2005), urethrotomy (Gera and Nigam, 1979) and tube cystostomy and urethrotomy. After taking a brief history, clinical signs and physiological parameters like heart rate (beats/minute), rectal temperature (°C) and respiratory rate (breaths/minute), colour of mucous membrane, skin tent test (Radostits et al., 2000), capillary refill time (seconds) were recorded. Intra–operative observations like status of urinary bladder (intact or ruptured), site of rupture of urinary bladder (dorsal neck or dorsal vertex/ ventral neck or ventral vertex/complete vs. subserous), appearance of urinary bladder surface (smooth vs. rough, inflamed, necrosed), adhesions of urinary bladder with peritoneum, omentum, mesentery or any other abdominal organs, if any, site of urethrotomy (post–scrotal vs. pre–scrotal or both), location of calculi (distal to sigmoid flexure or pre–scrotal region), number of incision made over urethra (1, 2, 3), catheterization of urethra (easy, difficult, very difficult or not possible) and urine flow from the Foley’s catheter and urethral catheter (free flow, drop by drop, flow upon pressure over caudal flank, no flow even upon pressure over caudal flank) etc. were recorded in buffalo calves treated with different techniques.
RRESULTS
The average age of calves was 4.86 months (3–10 months) with the maximum numbers of animals between 2 and 4 months (23/48; 47.92%) of age followed by the animals of 4–6 months (18/48; 37.5%), 6–8 months (5/48; 10.42%) and 8–10 months (2/48, 4.17%) of age. All the buffalo calves were uncastrated male. The average duration of urethral obstruction was 4.20 days (1–10 days). The clinical signs and body conditions varied according to the status of the urinary bladder and duration of urethral obstruction. Thirty four (70.83%) buffalo calves had fair general body condition, 13 (27.08%) buffalo calves had poor body condition and very poor or moribund general body condition was found in one case. The urinary bladder was found intact in 19 calves (39.58%) whereas in remaining 29 (60.42%) cases urine was present in the peritoneal cavity either due to complete rupture of the urinary bladder (16 cases) or subserous rupture of the urinary bladder (13 cases).
Clinical signs in the animals with intact urinary bladder included anuria, inappetance to anorexia, reluctant to walk, respiratory distress, prolonged recumbency, normal alert to depressed and dull appearance (2 cases), sunken eyes, rough to moist muzzle; inward and outward movement of the flank during straining, breath holding, engorged urethra, and anal sphincter movement, twitching of the penis, straining for urination, maintaining urinary posture for prolonged periods, tail lifting and swishing (Figure 1), licking of prepuce, adherence of sandy material at preputial hair (Figure 2) and preputial mucosa, restlessness, frequent attempt to urinate, prolapse of rectum and pain on palpation of penis/urethra.
In the cases with ruptured urinary bladder, anuria and bilateral distention of the abdomen i.e. water–belly was the most consistent clinical sign (Figure 3). The calves looked severely dull and depressed; however, few cases appeared alert and showed only mild degree of dehydration manifested with sunken eyes (sinking of eyeball into the socket) and rough body coat. In three cases, the eyes were severely sunken and ventro–lateral aspect of the orbital socket was visible. The calves with high degree of abdominal distension were reluctant to move forward and preferred to maintain standing posture. Two cases were brought to the clinics in recumbent position; they showed great discomfort and dyspnoea. In three cases, abdomen was so severely distended that fluid movement was seen in flank region while animals walked cautiously. The degree of dryness of muzzle, roughness of body coat, sunken eyes and ocular discharge were much severe than that of the calves that had intact urinary bladder. Edema of brisket and rectal prolapse were seen in one case each. In majority of the cases, palpebral mucosa was congested and gingival mucosa was pale and cold upon touching. Severe bradycardia (23 and 36 beats per minute) was seen in two cases and one of them that had the heart rate of 23 beats/minute, showed deep and loud heart sounds. Diarrhoea was seen in two cases; one of them had rectal prolapse.
The mean±SE capillary refill time (CRT) was 1.86±0.07 (1.06–3.48), which was within the normal reference range (1–2 sec.). The mean±SE time of skin fold test was 4.09±0.311 seconds (1.17 – 10.00), which was higher than the normal time. The extent of dehydration in buffalo calves varied from 4–10%. Thirty six calves had 6–8% dehydration, 7 calves had 4–6% dehydration and 5 calves had 8–10% dehydration.
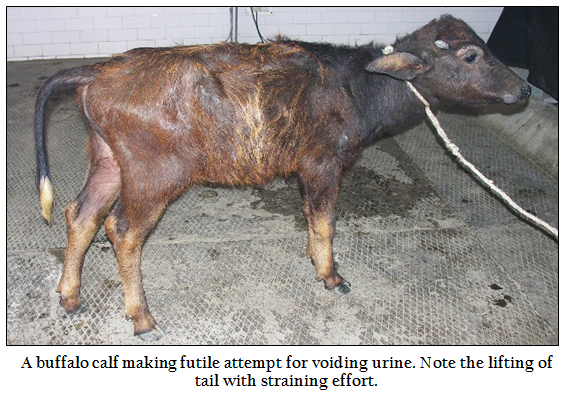
Figure 1: A buffalo calf making futile attempt for voiding urine. Note the lifting of tail with straining effort.
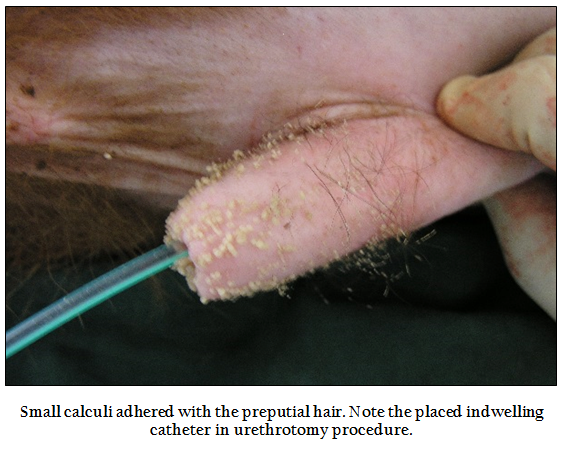
Figure 2: Small calculi adhered with the preputial hair. Note the placed indwelling catheter in urethrotomy procedure.
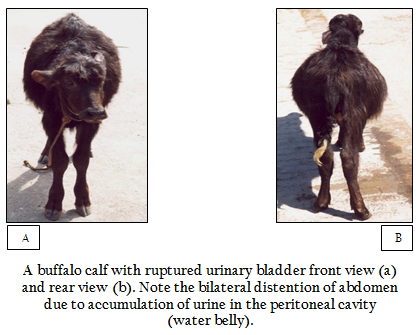
Figure 3: A buffalo calf with ruptured urinary bladder front view (a) and rear view (b). Note the bilateral distention of abdomen due to accumulation of urine in the peritoneal cavity (water belly).
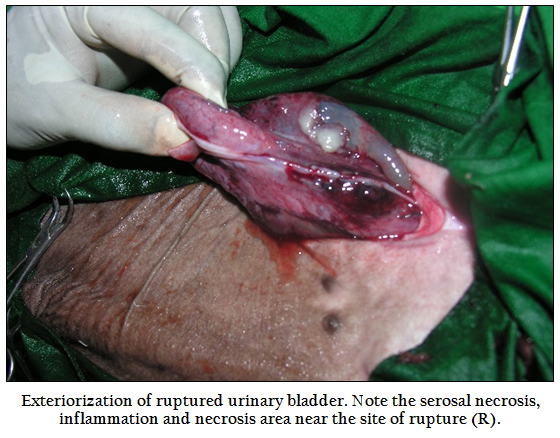
Figure 4: Exteriorization of ruptured urinary bladder. Note the serosal necrosis, inflammation and necrosis area near the site of rupture (R).
The mean±SE heart rate in the affected animals was 75.75±2.84 (23–128) beats per minute, which was higher than the normal reference value (66.00±2.91). The mean±SE respiratory rate (breaths/minute) was 30.62±2.00 (14–70), which was higher than the normal reference value (22.06±1.56). The mean±SE rectal temperature (°C) in the affected calves was 37.88±0.15 (34.50 – 40.30), which was within than the normal reference range (38.40±0.27).
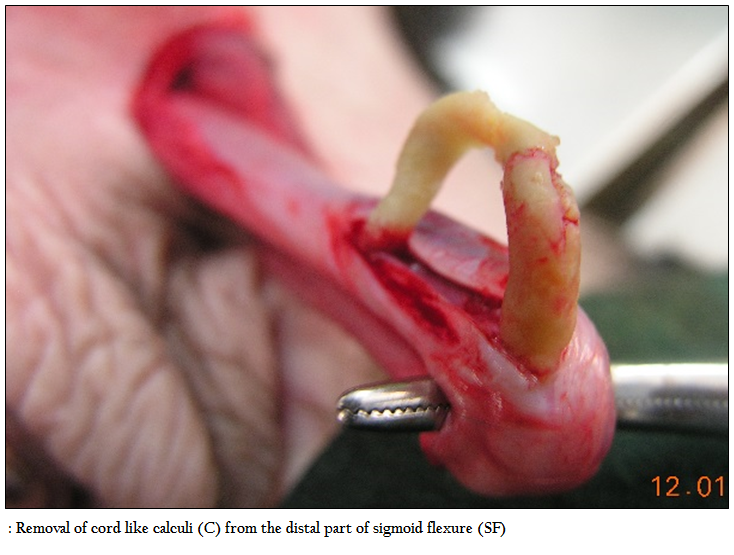
Urinary bladder was found intact in 19 buffalo calves and ruptured urinary bladder in remaining 29 calves. Amongst the ruptured urinary bladder, the site of rupture was complete and found on dorsum in 24 cases; out of which subserous rupture of urinary bladder was found in 13 buffalo calves, complete rupture towards neck region in 11 cases, at the ventral neck in 3 calves and in remaining 2 calves, rupture was seen at the vertex of the bladder. The appearance of the urinary bladder was smooth in 15 buffalo calves and the colour varied from pinkish to dark blue. In 25 buffalo calves, the surface of urinary bladder was rough, corrugated, inflamed and necrosed (Figure 4), some of them were chocolaty in colour. Out of 16 buffalo calves treated with urethrotomy only, eight calves had intact urinary bladder. Adhesion of urinary bladder with peritoneum, omentum or mesentery was seen in 19 calves (Figure 5) where as in remaining 21 calves bladder surface was free from adhesions. Thirty two buffalo calves in which urethrotomy (along with tube cystostomy; n=16, and urethrotomy alone; n=16) was performed, incision was made in post–scrotal region; over the sigmoid flexure in 21 calves, pre–scrotal in 4 calves, and in remaining 7 calves, both post and pre–scrotal incision was made. Calculi were located at distal to sigmoid flexure in 14 calves, pre–scrotal region in 13 calves and in remaining 5 calves, calculi were not retrieved. Calculi of different shape and consistency were removed in different animals whereas in one case, it was retrieved like a cord (Figure 6). For retrieval of sabulous calculi, single incision was made in 21 buffalo calves, two each in 6 calves and in remaining 5 calves, three incision was made to remove the all the calculi from the urethra. Catheterization into urethra was not possible in one case, easy in 19 cases, difficult in 8 cases and very difficult in 4 cases. Urine flow through urethral catheter was seen freely in 5 cases, upon abdominal pressure in 2 cases and drop by drop in 2 cases and in remaining 23 cases urine flow was not seen even upon abdominal pressure. In sixteen animals treated with tube cystostomy and urethrotomy, urine flow through Foley’s catheter was seen in all the sixteen cases. The average time of surgery for tube cystostomy was 31.44 minutes (15–60 minutes), for urethrotomy was 81.44 minutes (30–150 minutes) and both tube cystostomy and urethrotomy was 100 minutes (50–130 minutes).
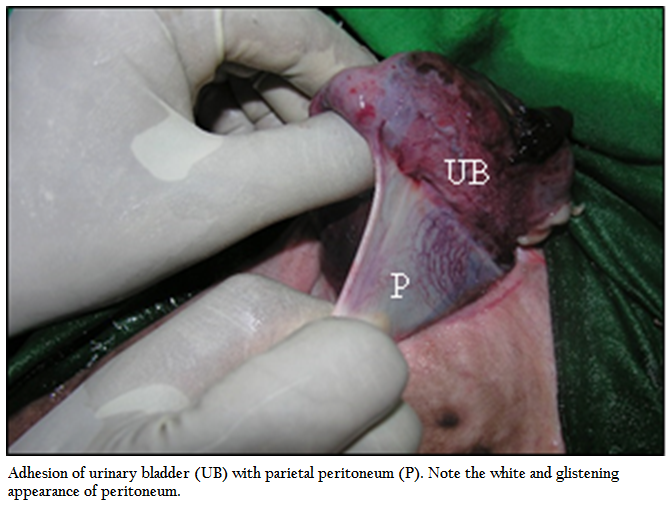
Figure 5: Adhesion of urinary bladder (UB) with parietal peritoneum (P). Note the white and glistening appearance of peritoneum.
DISCUSSION
The age of the buffalo calves varied from 3 months to 10 months (average age 4.86 months) with maximum calves aged between 2–4 and 4–6 months. Sharma et al. (2007) recorded about 60 per cent urethral obstruction occurs at an early age in ruminants. Gugjoo et al. (2013) reported that 84.61 per cent affected buffalo calves were of the age of 4–7 months. All the buffalo calves were male. Though, urolithiasis equally affects the male and female animals but obstruction occurs only in males (Larson, 1996; Radostitis et al., 2000) due to the presence of long and narrow urethra in males. The average duration of illness was 4.20 days (1–10). Similar findings were also reported by Amarpal et al. (2004) and Singh (2005).
The general body condition was mainly associated with the duration of illness and the status of urinary bladder (rupture or intact). General body condition was found fair in the cases that had illness for 1–3 days, poor to very poor in those cases that had illness for more than 4 day. Similarly, animals with intact urinary bladder were in better condition than those having rupture of urinary bladder. Rupture of urinary bladder may induce metabolic changes and the condition of the animals may deteriorate fast during the next 24–48 hours. The different clinical signs noticed with intact urinary bladder in the present study have also been noticed by various investigators (Monoghan and Boy, 1990; Radostitis et al., 2000; Walker and Hull, 1984). Monoghan and Boy (1990) opined that early signs of urethral obstruction are caused by the urethral pain. Pulsation of urethra and rectal prolapse could probably be due to continuous efforts made for the urination. When urinary bladder ruptures, initial pain relief occurs, and clinical signs may not appear for 1 to 2 days (Monoghan and Boy, 1990; Radostits et al., 2000). However, over the course of the next few days, uraemia becomes severe, and the animal progresses to severe depression, anorexia and dehydration. Ventral abdominal distention may be noticed (water belly) and fluid may be detected by ballottement of the abdomen. In the present study, almost similar types of clinical signs with great variation in their degree were recorded in 29 cases of ruptured urinary bladder. Severe dehydration in these cases could be due to shifting of water from the interstitial and intravascular compartment into the peritoneal cavity (Radostitis et al., 2000). In case of high degree of abdominal distension calves were reluctant to move forward and preferred to maintain standing posture to avoid the pressure over the diaphragm and minimize respiratory distress. Mean heart rate was higher than the normal reference value in most of the cases in the present study. Increased heart rate has also been observed by various earlier workers in the cases of obstructive urolithiasis (Jadon et al., 1987; Joshi et al., 1989; Monoghan and Boy, 1990; Tsuchiya and Sato, 1990; Singh, 2005). Accumulation of toxic metabolic waste products in the uremic animals was suggested to be one of the causes for the increased heart rate (Lavania et al., 1973). Monoghan and Boy (1990) attributed the increased heart rate in obstructive urolithiasis to pain and the progressive systemic disturbances. (Kelly et al. 1984) considered dehydration, biochemical alterations, intercompartmental fluid shifts and myocardial ischaemia as the prime causes of increased heart rate. Inappetance and prolonged duration of illness and myocardial asthenia resulting from hyponatremia and hyperkalemia in ruminants (Radostits et al., 2000) were suggested as the possible causes of increased HR in the cases of obstructive urolithiasis. However, Gangwar et al. (1990) found decreased heart rate in the cases of experimental urolithiasis. The stage of uraemia or duration of illness could be the factors responsible for variation in the heart rate.
Respiratory rate was higher than the normal reference value, which could be due to pain caused by urethral calculi, abdominal crisis, electrolyte aberrations like hypocalcaemia, hypomagnesaemia and hypovolumic shock (Wilson and Lofstedt, 1990). Increased respiratory rate has been reported earlier in uraemic buffalo calves by (Jadon et al. 1987), in sheep by (Joshi et al. 1989) and in goats by (Tsuchiya and Sato 1990).
The rectal temperature was within the normal reference range However, rectal temperature ranged from 34.50 – 40.30°C. The variation in the RT could probably be due to the variation in the stage of uraemia, duration of illness and degree of haemodynamic changes. Sockett et al. (1986) and Gangwar et al. (1990) found no change in the values of RT after experimental creation of urethral obstruction, while (Kulkarni et al. 1985) recorded variable RT in calves.
The capillary refill time (CRT) was within the normal reference range. CRT is the indirect measurement of tissue perfusion. Tissue perfusion may be decreased when the gums are pale, rather than pink and the capillary refill time exceeds 1.5 seconds (Hall et al., 2001). Most of the buffalo calves might be having compromised tissue perfusion due to reduction in circulating blood volume as a result of dehydration. The skin tent test (STT) and per cent dehydration are closely related to each other as the loss of skin elasticity is primarily due to loss of fluid from the interstitial and intracellular spaces (Radostitis et al., 2000). The per cent dehydration varied from 4 to 10% (STT 1.17 – 10.00 sec.) with most of the calves having 6–8% dehydration. However, per cent dehydration was more in the cases of ruptured urinary bladder, which might be due to shifting of more water from the interstitial and intravascular fluid into the peritoneal cavity (Donecker and Bellamy, 1982).
Status of urinary bladder was found intact in 19 calves and ruptured in remaining 29 buffalo calves. Number of cases with rupture of urinary bladder was more because of late presentation of the cases at the clinics, treatment with furosemide (Lasix; Sanofi Aventis, Aventis House, Andheri–E, Mumbai–400 093) before presentation, and complete obstruction caused by sabulous calculi (Monoghan and Boy, 1990; Singh and Singh, 1990; Amarpal et al., 2004). The site of rupture varied from dorsum to ventral aspect and vertex to neck of the bladder. The exact reason for pattern of rupture of urinary bladder was not clear but it could be due to weakening of bladder muscles and transitional epithelial on dorsal side. Monoghan and Boy (1990) also observed that most of the rupture was seen on dorsal side. Incomplete rupture or subseruous rupture of urinary bladder in buffalo calves could be due to separation of muscles rather than complete tearing.
Smooth bladder surface and pinkish to blue colouration of the bladder in 15 buffalo calves having intact bladder could be due to over stretched bladder wall resulting in venous congestion. Rough, corrugated, inflamed and necrosed bladder recorded in 25 cases might be due to fact that after rupture of urinary bladder it tends to contract leading to rough appearance. Adhesion of ruptured urinary bladder in 19 cases was probably because of rough bladder surface which was attached with omentum, peritoneum and even mesentery. Thirty two buffalo calves in which urethrotomy (urethrotomy; n=16 and tube cystostomy plus urethrotomy; n=16) was performed, incision was made in post–scrotal region over the sigmoid flexure in 21 calves, pre–scrotal in 4 calves and in remaining 7 calves, both post and pre–scrotal incision was made. The urethrotomy incision through sigmoid flexure was based on the fact that it was the most common site of calculi lodgment (Gera and Nigam, 1979; Radostits et al., 2000). Incision was made both in pre and post scrotal region because of presence of calculi at both site. In four cases, calculi were easily palpable at pre–scrotal region and hence incision was made over there. Calculi were located at distal to sigmoid flexure in 14 calves, pre–scrotal region in 13 calves and in remaining 5 calves, calculi could not be retrieved. Although incision was made in the skin over post–scrotal incision but in seven cases incision was made in pre–scrotal region of penis after pulling through the post–scrotal region. In most of the cases calculi were removed in different stages whereas in one case, it was retrieved like a cord. In buffalo calves, most of the calculi present were sandy in nature and occupied long distance contrarily to adult buffalo or bullock where single calculus was the cause of obstruction (Sharma and Singh, 2001). For retrieval of sabulous calculi, single incision was made in 21 buffalo calves, two in 6 calves and in remaining 5 calves, three incisions were made to remove the whole calculi from the urethra due to variation in location of calculi. Catheterization into urethra was not possible in one case where urethrostomy had to perform; however, it was easy in 19 cases, difficult in 8 cases and very difficult in 4 cases. The catheterization was particularly difficult or very difficult in those cases where two or three incisions were made to remove the calculi. Urine flow through urethral catheter not seen in most of the cases. Under epidural analgesia urine flow depends upon passive flow of urine. In the buffalo calves the catheterization was not complete and it was done only up to the ischial arch due to presence of dorsal urethral diverticulum (Larson, 1996; Radostits et al., 2000). Closed urethral sphincter could have not allowed free flow of urine in those cases. In sixteen animals treated with tube cystostomy and urethrotomy, urine flow through Foley’s catheter was seen in all the cases and no urine flow was noticed through urethral catheter which could be due to rapid and dependent flow of urine through Foley’s catheter.
The average time of surgery for tube cystostomy was 31.44 minutes (15–60 minutes), for urethrotomy was 81.44 minutes (30–150 minutes) and both tube cystostomy and urethrotomy was 100 minutes (50–130 minutes). Tube cystostomy was a fast and easier procedure particularly in intact urinary bladder where Foley’s catheter could be inserted bluntly into the urinary bladder. Urethrotomy took longer time for surgery than the tube cystostomy because of difficulty in indwelling catheterization due to variable diameter of the urethra against constant diameter of the catheter, variation in the amount of lodged calculi, and damage or inflammation to urethra etc. The time of surgery was maximum in combination group as two procedures were performed together.
REFERENCES
Amarpal, Kinjavdekar P, Aithal HP, Pawde AM, Singh T, Pratap K (2004). Incidence of urolithiasis: A retrospective study of five years. Indian J. Anim. Sci. 74: 175 – 177.
Donecker JM, Bellamy JEC (1982). Blood Chemical Abnormalities in Cattle with ruptured Bladders and Ruptured Urethras. Can. Vet. J. 23: 355 –357.
PMid:17422206 PMCid:PMC1790279
Gangwar S D, Pandey NN, Celly CS (1990). Clinico–haematological profile of calves in experimental uraemia of post–renal origin. Indian Vet. J. 67: 645 – 648.
Gera KL, Nigam JM (1979). Urolithiasis in Bovines: A report of 193 clinical cases. Indian vet. J. 56: 417 – 423.
Gugjoo MB, Zama MMS, Amarpal, Mohsina A, Saxena AC, Sarode IP (2013). Obstructive urolithiasis in buffalo calves and goats: incidence and management. J. Adv. Vet. Res. 3: 109 – 113.
Hall LW, Clarke KW, Trim CM (2001). Patient monitoring and clinical measurement, In: Veterinary Anaesthesia. W. B. Saunders, Philadelphia. 39.
http://dx.doi.org/10.1016/B978-070202035-3.50003-6
Jadon NS, Prakash P, Joshi HC, Kumar A (1987). Clinico–haematological changes in experimental urethral obstruction in buffalo calves. Indian J. Vet. Med. 7: 49 – 51.
Joshi HC, Zangana IK, Saleem AN (1989). Haemato–biochemical and electrocardiographic changes in uraemia in sheep. Indian J. Vet. Med. 9: 95 – 99.
Kelly WR (1984). The abdominal and associated digestive organs. In: Veterinary clinical diagnosis. Bailliere Tindall, London, 147 – 213.
PMid:6543247
Kulkarni BB, Chandna IS, Peshin PK, Singh AP, Singh J (1985). Experimental evaluation of treatment of uraemia due to ruptured urinary bladder in calves–correction of acid–base and electrolyte imbalance and water deficit. Indian J. Vet. Surg. 6: 25 – 30.
Kushwaha RB, Gupta AK, Dwivedi DK, Sharma A (2011). Obstructive urolithiasis in Small Ruminants and its surgical management. Intas Polivet 12: 359 – 62.
Larson BL (1996). Identifying, treating, and preventing bovine urolithiasis. Vet. Med. 91: 366 – 377.
Lavania JP, Misra SS, Angelo SJ (1973). Repair of rupture of bladder in a calf–a clinical case. Indian Vet. J. 50: 477 – 481.
Monoghan ML, Boy MG (1990). Ruminants Renal System: Diseases of the renal system. In: Large Animal Internal Medicine. C. V. S. Mosby Company, Philadelphia, Toranto, 888 – 890.
Radostits OM, Blood DC, Gay CC, Hinchcliff KW (2000). Veterinary Medicine: A textbook of the diseases of cattle, sheep, pigs, goats and horse. Bailliere Tindall, London. 1877.
Sharma AK, Mogha IV, Singh GR, Amarpal, Aithal HP (2007). Incidence of urethral obstruction in animals. Indian J. Anim. Sci. 77: 455 – 56.
Sharma SN, Singh J (2001). The Urinary System. In: Ruminant Surgery, CBS Publishers and distrubuters, New Delhi, 263 – 273.
Singh J, Singh K (1990). Obstructive urolithiasis and uraemia in cattle and buffaloes– A review. Indian J. Vet. Surg. 11: 1 – 20.
Singh, T (2005). Studies on aetiopathogenesis and surgical management of urolithiasis in goats. PhD thesis, submitted to Deemed University, IVRI, Izatnagar (UP) –143 122.
Sockett DC, Knight AP, Fettman MJ, Kiehl AR, Smith JA, Arnold SM (1986). Metabolic changes due to experimentally induced rupture of the bovine urinary bladder. Cornell Vet. 76: 198 –212.
PMid:3698602
Tsuchiya R, Sato M (1990). Uraemic changes induced by experimental urinary retention in goats. Japanese J. Vet. Sci. 52: 113 – 119.
http://dx.doi.org/10.1292/jvms1939.52.113
Van Metre D (2004). Urolithiasis. In: Farm Animal Surgery, W. B. Saunders, New Yark, 534 – 547.
Walker DF, Hull BL (1984). Bovine urogenital surgery. In: The Practice of Large Animal Surgery, W. B. Saunders, Philadelphia, 1042 – 1080.
Wilson WD, Lofstedt J (1990). Alterations in respiratory functions. In: Large Animal Internal Medicine, C. V. S. Mosby Company, Philadelphia, 47 –99.