Advances in Animal and Veterinary Sciences
Research Article
Evaluation of Penile Blood Flow in Dogs With TVT Before and After Chemotherapeutic Treatment With Special Reference to its Angioarchitecture
Elshymaa A. Abdelnaby1, Yara S. Abouelela2*, Noha A. E. Yasin3
1Theriogenology Department, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt; 2Anatomy and Embryology Department, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt; 3Cytology and Histology Department, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt.
Abstract | Canine transmissible venereal tumor (CTVT) is a coitally transmitted neoplasm with the prevalent distribution. This study aimed to compare Doppler changes of internal pudendal (I Pu.A), dorsal (Do Pe. A), and deep penile arteries (De Pe. A) in dogs suffering from TVT and confirm its manner by anatomical vascular segmentation of the penis. Thirty males were categorized into two groups; control groups (Group I, n=17) intact adult healthy dogs of different breeds and TVT affected dogs (Group II, n=13). Diagnosis of TVT was manifested by gross clinical indicators, histopathological, and Doppler examination. All stud dogs were observed by Doppler twice per week for 14 days before and after intravenous treatment by vincristine at a dosage of 0.025 mg/kg body weight. I Pu.A, Do Pe. A and De Pe.A cross-sectional diameters were increased during the first week of infection with a slight increase in the second week, but after treatment, the diameters were showed a consecutive decline in the first two weeks. All arteries Doppler indices were decreased significantly (P ≤ 0.05) in the first and second weeks of infection but in the first week after treatment, the indices were elevated significantly with a marked increase in the second week when compared to normal and to those infected ones. All arteries’ blood flow rate were increased significantly (P ≤ 0.05) in the two weeks of TVT infection, but after vincristine administration, the blood flow rate was significantly decreased. It was concluded that Doppler could be used as a diagnostic tool for dogs suffering from venereal tumors by evaluating the hemodynamic changes in internal pudendal and penile arteries blood flow velocities waveform pattern.
Keywords | Transmissible venereal tumor; Dogs; Doppler; Pudendal artery; Penile artery
Received | April 02, 2021; Accepted | April 17, 2021; Published | July 01, 2021
*Correspondence | Yara S Abouelela, Anatomy and Embryology Department, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt; Email: yarasayed89@gmail.com
Citation | Abdelnaby EA, Abouelela YS, Yasin NAE (2021). Evaluation of penile blood flow in dogs with tvt before and after chemotherapeutic treatment with special reference to its angioarchitecture. Adv. Anim. Vet. Sci. 9(8): 1159-1168.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.8.1159.1168
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Abouelela et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
A transmissible venereal tumor (TVT), also called transmissible lymphosarcoma or Sticker tumor (Milo and Snead, 2014), is a benign tumor of the male dog that mainly affects the dog’s overall genitalia as well as affects the penile erection. As it is transmitted during the coitus (Tella, 2004), besides, it occurs in sexually mature animals (Zayas et al., 2019) during coitus which is transplanted with viable cells across complex histocompatibility barriers within the same species (Mukaratirwa and Gruys, 2003) and can be also transmitted to other canine family members (Abeka YT, 2019).
Diagnosis of this venereal disease is based on the case history, clinical manifestation, and cytological findings. Cytology is usually a very rapid and field diagnostic method but the biopsy for histopathological examination is considered the most dependable technique for diagnosis (Thangathurai et al., 2008; Nak et al., 2005). Clinically, the disease started with inflammation of mucosa then the presence of small nodules that changed rapidly into red which becomes either single or multiple nodules (Milo and Snead, 2014). Macroscopically, the tumor could appear nodular or papillary, and firm in consistency with a diameter up to 15 cm (Hiblu et al., 2019; Baba and Câtoi, 2007). Histologically, tumor cells are uniformly ovoid, round, or slightly polyhedral, with eosinophilic cytoplasm and large round nuclei (Sharma et al., 2017).
Several techniques including surgery, immunotherapy, radiotherapy, biotherapy, and chemotherapy have been applied for TVT treatment (Purohit, 2008). Chemotherapy is the most effective and practical therapy for the treatment as it provides a good prognosis in CTVT cases (Abeka, 2019; Uçmak et al., 2019). Vincristine sulfate (VCR), a natural vinca alkaloid mitosis inhibitor isolated from the Madagascar periwinkle plant (Catharanthus roseus), is professionally used as a chemotherapeutic drug for the treatment of Canine TVT worldwide (Bates and Eastman, 2017; Özalp et al., 2012). Vincristine is administered with a constant dose of 0.025 mg/kg, IV weekly (Boscos and Ververidis, 2004). The treatment is almost gradual and obvious as the accurate treatment began with a cure rate reaching 100% (Hantrakul et al., 2014). The use of Doppler technology in human medicine for the evaluation of testicular and penile vascularization is already well described (Altinbas and Hamidi, 2018; Fernandes et al., 2018; Lotti F and Maggi, 2017). In humans, it is mainly used for accurate diagnosis of testicular torsion, varicocele (Schurich et al., 2009), and azoospermia diagnosis, which may be related to many infertility problems, as well as penile dysfunction like abnormal erection (Altinbas and Hamidi, 2018).
In veterinary medicine, many reports studied the testicular Doppler ultrasound in equines (Pozor and McDonnell, 2004; Abdelnaby et al., 2021a), camels (Kutzler et al., 2011) and dogs (Zelli et al., 2013; Carrillo et al., 2012). In dogs, some studies also reported the testicular artery’s blood supply and its course around pampiniform plexus marginally (Gumbsch et al., 2002; Günzel-Apel et al., 2001). The combination of grey ultrasonography and color Doppler imaging is well accepted as a perfect technique for the evaluation of scrotal lesions and genital organ vascularization in dogs (Günzel-Apel et al., 2001). However, the establishment of an appropriate Doppler ultrasonographic parameter is important to measure the normal penile parameters in dog species and abnormal penile Doppler parameters in the case of dogs infected with TVT. Due to the scarcity of studies on this subject, this study aimed to associate Doppler parameters of the internal pudendal artery in addition to penile arteries measured in different regions of the dog penis and to compare these parameters between animals before and after vincristine treatment which affirmed by studying the angioarchitecture of the penile arteries (Adams, 2004).
Materials and Methods
Animals and housing
All animals were treated with an approved protocol in accordance with the Institutional Animal Care and Use Committee (IACUC) of the Faculty of Veterinary Medicine, Cairo University (with protocol no. Vet CU16072020187). Thirty male dogs; (nine German shepherds, seven golden retriever, seven boxer breed average weight 27 kg and age 8-9 years, and seven Fox Terriers, the average weight of 9 kg and age 2-3 years) were examined. Animals were categorized into two main groups; control normal group (Gp I) (seventeen male dogs; four German shepherds, four golden retrievers, four boxer and five fox terriers) and group II (Gp II) (thirteen infected male, five German shepherds, three golden retriever, three boxers and two fox terrier) suffered from TVT. Four animals from Gp I (one German shepherd, one golden retriever, one boxer breed, and one Fox Terriers) were used for vascular architectures to ensure that there no significant difference between different breeds in their arterial vasculatures. The German and golden dogs arrived in the pet’s clinic at Theriogenology Department, whereas the fox Terriers were stud dogs that belonged to the breeder in a private place. All dogs were exposed to the same environment during semen collection (for example, transportation to hospital with similar cohorts’ presents including estrus females). No changes in environmental conditions that affect heart rate, blood pressure, and alter vascular indices.
Vascular architectures
Animals that used for vascular architectures were arrived at the clinic recently dead within two hours by accidental or clinical diseased conditions with normal genitalia, the abdominal cavities of these animals were opened, after that, the abdominal aorta was exposed to catheterize and removed the clotted blood by warm saline solution (Reem et al., 2021; El-Karmoty et al, 2017). then injected by previously prepared red latex (white latex neoprene mixed with red Rotring ink) (El-Bably and Abouelela, 2021), then kept for 3-5 days at room temperature in formalin solution 10% to solidify the latex (Aril et al., 2011) after that the dissection of internal pudendal artery was began. Digital camera was applied for snapping photos.
Clinical manifestation
Dogs from Gp II were excessively licking the affected area. The disease located on the glans penis was associated with pain, intermittent hemorrhages, and thickening of the tissue. It appeared as an irregular mass, in the form of cauliflower-like nodules, friable, red to flesh-colored, and bleeds easily on manipulation. The penis naturally protruded without difficulty, but the tumor mass made the preputial opening relatively small. The diagnosis was confirmed by histopathological examination of hematoxylin and eosin-stained sections of the tumor mass.
Histopathological examination
Biopsies were obtained from Gp II for the histopathological examination of the tumor mass. Samples were fixed in 10% neutral buffered formalin (NBF) for 48 hours. After routine dehydration with various ascending gradients of ethyl alcohol, samples were cleared by xylene and finally embedded in paraffin wax. Four to five µm-thick sections were subjected to hematoxylin and eosin (H and E) stain (Bancroft and Gamble, 2008). The stained slides were examined using the light microscope (LEICA DM500), then the images were captured with a camera attached to the microscope (LEICA ICC50 HD) and finally examined by image analysis software (LEICA microsystems (LAS version 3.8.0 (build:878), Leica Ltd) image analyzer computer system) at Cytology and Histology Department, Faculty of Veterinary Medicine, Cairo University.
Ultrasonography
All normal animals examined twice weekly before semen collection to assist the vascularity of internal pudendal artery, dorsal and deep penile arteries, but dogs suffered from TVT examined twice /week at first and second week before treatment and then examined twice /week at the first and second week after treatment by vincristine. All dogs were examined in dorsal recumbence, low abdomen and acoustic coupling gel was applied at the penis. The time frame from the identification of diseased state to first measurement and subsequent treatment is actually a four-week time frame, i.e. week one and two of infection, then week one and two of treatment. The initial ultrasound examinations were performed at the time of penile scraping, ultrasound examination was performed using B‐mode, color, and spectral modes Doppler ultrasound (US) for internal pudendal artery, dorsal and deep penile arteries. A spectral-wave Doppler equipped with 7.5 MHz linear-array transducer (Power: Max (100%; Maher et al., 2020a; 2020b), pulse repetition frequency: 3.3 kHz, optimization of color and power Doppler parameters to detect the presence of slow flows, Doppler angle: 45 °, two color map, and Brightness: 80%) (EXAGO, France) was used by the color and spectral modes (Abdelnaby and Abo El‐Maaty, 2020; Abdelnaby and Abo El‐Maaty, 2021). All measurements were taken in a standard location along the vessel with three multiple measurements taken from each dog. Both arteries were examined along two planes, longitudinal and transverse axes. For each plane, multiple static colored and normal grey images were obtained (Carreira and Martins-Bessa, 2008). Color and spectral Doppler parameters were optimized to detect the internal pudendal, dorsal and deep arteries blood flow velocities waveform by estimating the changes in Doppler parameters (Kocako et al, 2007). Doppler indices were estimated as in cows (Abdelnaby et al., 2018; Abdelnaby, 2020a; Abdelnaby et al., 2021b), mares (Abdelnaby et al., 2017a; Abdelnaby et al., 2016; Abdelnaby et al., 2020) and ewes (Abdelnaby, 2020b). Resistive index (RI), pulsatility index (PI), blood flow rate /bpm were measured in the normal dogs, and those suffering from TVT.
Chemotherapeutic treatment
All infected dogs (n=13) were received chemotherapeutic agent, the treatment was started with vincristine sulfate (Vincristine®, Gedeon Richter, Hungary) at a dosage of 0.025 mg/kg body weight (BW) intravenously once a week in saline fluid therapy 500 ml and continued with three treatments of vincristine sulfate at the same dosage once a week, it can be interpreted that an initial dose was given and then continued with three additional weekly doses (Boscos and Ververidis, 2004). Control animals were similarly treated with saline infusion and measured at similar intervals to affected animals. There was no recurrence of any tumors observed for six months of monitoring since the last injection. After the end of treatment, the blood supply of arteries was also monitored during the first and second weeks.
Statistical analysis
Statistical analysis of color Doppler findings was performed using software SPSS. Doppler velocity rate and indices were calculated by the ultrasound equipment’s algorithm package based on three waveforms for each measurement. ANOVA test was used for the analysis of variance. The multiple range test for Duncan was obtained. The level of significance was set at P ≤ 0.05.
Results
Anatomical finding of pudendal and penile arteries
The arterial supply of the penis was originating from the internal pudendal artery (Figure 1A/6) as the artery of the penis, which released urethral branch then entered the penial tissue by three branches; the artery of the bulb of the penis (Figure 1B/10) entered the bulb of penis and nourished the corpus spongiosum penis till reaching the pars longus as well as the penial urethra, the second one was the deep artery of penis which supplied the penial corpus cavernosum, finally the dorsal artery of penis (Figure 1C/12) which ran along the dorsolateral face of the erectile tissue and gave three branches just before the pars bulbus glandis; preputial branch for prepuce (Figure 1D/ a), superficial branch for bulbus glandis (Figure 1D/ b), the deep branch (Figure 1D/ c) entered in between the erectile tissue and extended within the pars longa glandis till reaching the tip of the penis.
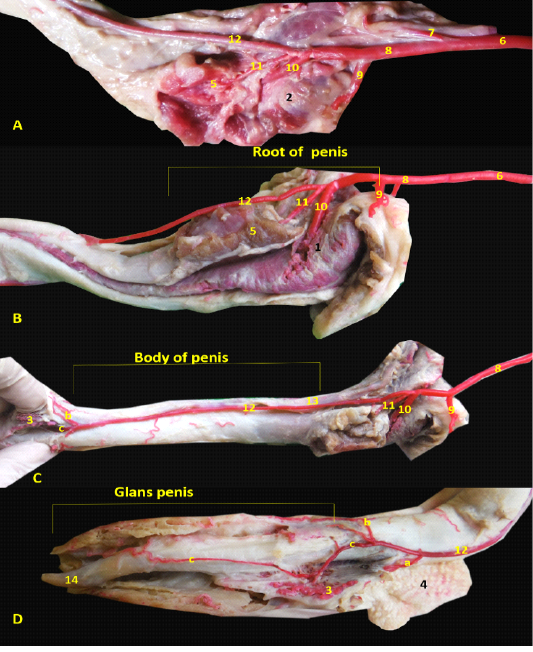
Figure 1: Images showed the distribution of the internal pudendal and penial arteries inside the penial tissue A- Origin of penial artery. B- branches of penial artery at penial root, C- branches of dorsal penial artery in penial body, D- dorsal penial artery at glans penis. 1- bulb of the penis, 2- bulbospongiosus muscle, 3- bulbus glandis, 4- prepuce, 5- ischiocavernosum muscle , 6- internal pudendal artery, 7- caudal rectal artery, 8- penial artery of the penis, 9-uretheral artery, 10- the artery of the bulb of the penis, 11- deep artery of penis, 12- dorsal artery of penis, a- preputial br., b- superficial br., c- deep br., 13- dorsal artery of penis, 14- fibrocartilaginous tip of Os-penis.
Macroscopic diagnosis
Grossly, the tumor masses were irregular, reddish cauliflower-like nodular overgrowth on the glans penis. The consistency of tumor mass was soft and tended to bleed (Figure 2A and 2B). On the other hand, the penis partially returned to the normal shape with an appreciable reduction in lesions in the first week after treatment by vincristine (Figure 2C) while complete return occurred in the second week after treatment (Figure 2D).
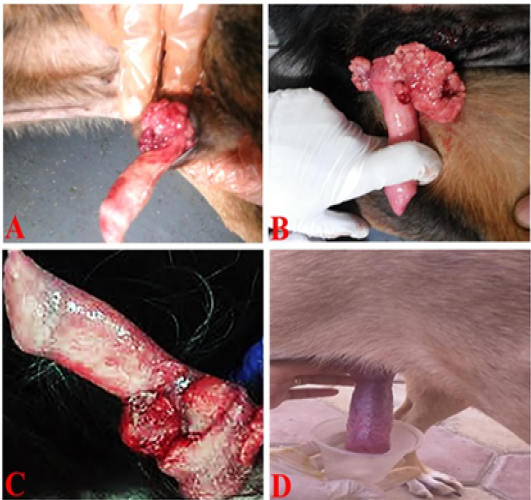 Figure 2: TVT and its response to vincristine therapy in male dogs. (A and B) showed an irregular, large, friable, reddish TVT mass (multinodular, cauliflower-like mass) overgrowth on the glans penis. (C) One-week post-treatment with the vincristine sulfate, the lesion presented an appreciable reduction. (D) By the end of the second week of vincristine therapy, the tumor mass had completely regressed.
Figure 2: TVT and its response to vincristine therapy in male dogs. (A and B) showed an irregular, large, friable, reddish TVT mass (multinodular, cauliflower-like mass) overgrowth on the glans penis. (C) One-week post-treatment with the vincristine sulfate, the lesion presented an appreciable reduction. (D) By the end of the second week of vincristine therapy, the tumor mass had completely regressed.
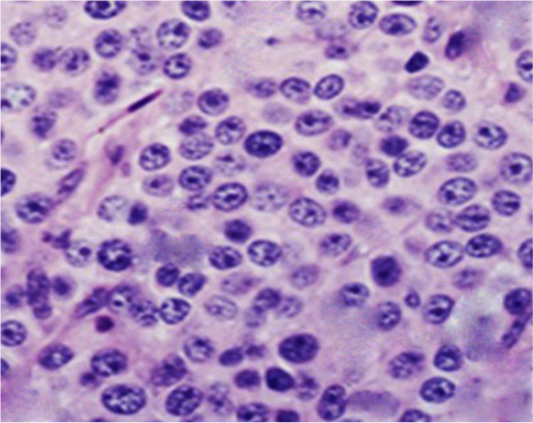
Figure 3: Photomicrograph showing histopathological analysis of biopsies from infected TVT dogs (Gp II). The neoplastic cells were numerous, round to oval, of relatively uniform size with indistinct outlines growing in a scanty fibrous stroma. The cells had lightly eosinophilic granular cytoplasm and large, round to ovoid nuclei with prominent nucleoli. Mitotic figures were also plentiful (H&E, X400).
Histopathology
Histopathological investigations of neoplastic nodules from Gp II revealed hypercellularity with indistinct cellular outlines. The neoplastic cells were large (of a relatively uniform size), round to oval often growing in sheets within a scanty stromal tissue (thin fibrous connective tissue). The tumor cells possessed large round to oval vesicular nuclei with prominent single nucleoli. Few cells revealed coarse chromatin. The cytoplasm was slightly granular, vacuolated, and eosinophilic. Mitotic figures were also present (Figure 3).
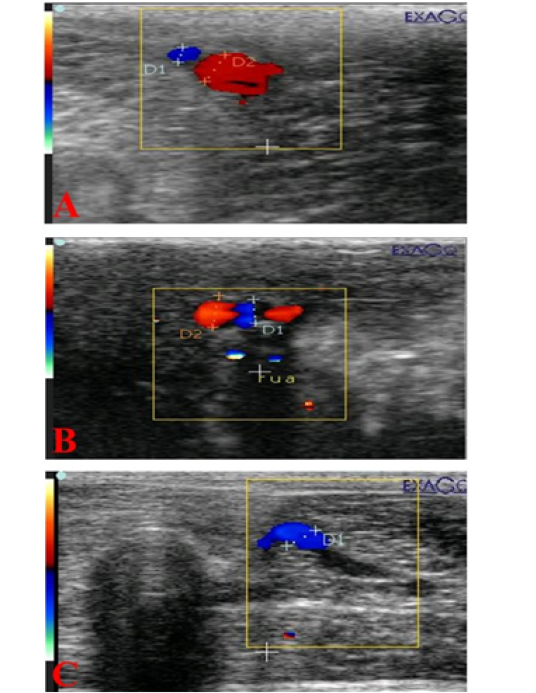
Figure 4: Ultrasonograms showing internal pudendal artery (I Pu.A.) (A), dorsal penile artery (Do Pe.A) (B), and deep penile artery (De Pe.A) (C), diameters (mm) in dogs suffered from TVT.
Cross-sectional diameter (mm) of pudendal and penile arteries
The mean cross-sectional diameter of the internal pudendal artery (I Pu.A.) showed a significant (P ≤ 0.05) high value during the first week of infection (Figure 4A; Figure 5A) and then showed a slight increase in the second week, but after administration of chemotherapeutic drug the diameter showed a marked significant (P ≤ 0.05) decrease in the first week after injection and another decrease in the second week compared to the control and the diseased group before treatment. In addition to, both dorsal (Do Pe. A) and deep penile arteries (De Pe. A) cross-sectional diameters (Figure 4B, 4C) increased significantly (P ≤ 0.05) in the infected group (Figure 5B, 5C) during the first week of infection when compared to the normal control group and then increased again in the second week, but after vincristine drug, the diameter showed a significant (P ≤ 0.05) marked decrease in the first week after injection and another decrease in the second week compared to the control and the diseased group before treatment.
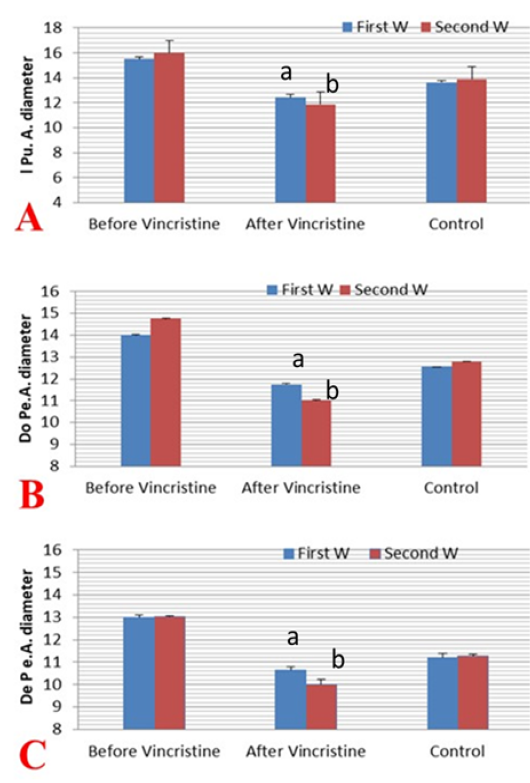
Figure 5: Mean ±SD internal pudendal artery (I Pu.A) (A), dorsal penile artery (Do Pe.A) (B), and deep penile artery (De Pe.A) (C) cross-sectional diameters (mm) during the first and second week before and after treatment by vincristine compared to control group.
Doppler indices of pudendal and penile arteries
The resistance index (RI) and pulsatility index (PI) were measured in the first two weeks before and after treatment by vincristine and compared to the normal control group (Figure 6 A-C). The I Pu.A RI was decreased significantly (P ≤ 0.05) in the first and second week of infection but the value more declined in the second week when compared to the normal dogs, but in the first week after administration of the drug the RI value of I Pu.A increased significantly (P ≤ 0.05) and showed a marked elevation in the second week after treatment when compared to normal and to those infected (Figure 6A). The two Doppler indices were also evaluated in the Do Pe. A (Figure 6B), the RI and PI of this artery was decreased significantly (P ≤ 0.05) in the
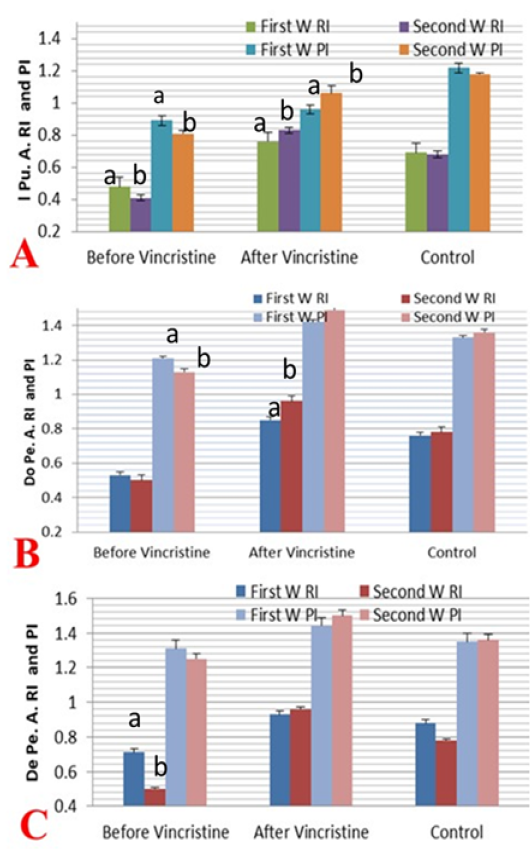
Figure 6: Mean ± SD internal pudendal artery (I Pu.A.) (A), dorsal penile artery (Do Pe.A) (B), and deep penile artery (De Pe.A) (C) resistance index (RI) and pulsatility index (PI) during the first and second week before and after treatment by vincristine compared to control group.
first two weeks of infection by TVT when compared to normal values in normal dogs, but in the first two weeks after administration, Doppler indices were increased significantly (P ≤ 0.05) with a linear marked elevation in the second week after treatment, as compared to normal and those infected. As shown in (Figure 6C) both Doppler indices in the De Pe. A were measured in those infected and normal dogs. The RI and PI of this deep artery were decreased significantly (P ≤ 0.05) in the first two weeks of infection by TVT when compared to normal values in normal dogs, but the level of decline was more pronounced in the second week than the first week of infection, but in the first two weeks after chemotherapy administration. Both Doppler indices values were increased significantly (P ≤ 0.05) and showed a marked elevation in the second week than the first week after treatment, as compared to normal and those infected.
Rate of blood flow of pudendal and penile arteries
I Pu.A rate of blood flow (bpm) was increased significantly (P ≤ 0.05) in the first and second week of TVT infection when compared to normal before any administration of a chemotherapeutic drug, but after vincristine administration, the blood flow rate was significantly (P ≤ 0.05) decreased when compared to the rate before treatment which indicates that the blood flow rate was associated with the decrease of the velocity of the blood flow which reflected to the vascular resistance of the artery. The Do Pe.A blood flow rate also increased in the first two weeks of infection, but after vincristine injection, the blood flow rate of this artery decreased significantly (P ≤ 0.05) when compared to that before injection (Figure 7). Finally, the blood flow rate in the De PeA. was increased significantly (P ≤ 0.05) in the first and second week of TVT infection when compared to normal before any administration of a chemotherapeutic drug, but after vincristine administration, the rate of blood flow was significantly (P ≤ 0.05) decreased when compared to the rate before treatment.

Figure 7: Mean ± SD internal pudendal artery (I Pu.A), dorsal penile artery (Do Pe.A), and deep penile artery (De Pe.A) blood flow rate (bpm) during the first and second week before and after treatment by vincristine compared to control group.
Discussion
In our study the internal pudendal vessel extends as artery of the penis that is the principal vessel of the penis with its branches (Adams, 2004; Slatter and Boothe, 2003), the dorsal artery arborizes at the level of pars bulbus glandis into three branches (Smith, 1999). TVT is found in both tropical and subtropical climates, particularly in a large population of poorly controlled, free-roaming dogs (Setthawongsin et al., 2019; Das and Das, 2000). So, stray dogs and free-roaming owned dogs are the major populations of TVT (Setthawongsin et al., 2019). TVT is more widely popular in intact young to adult dogs (Setthawongsin et al., 2019; Amaral et al., 2007) than older ones (Ganguly et al., 2016).
Exfoliation and transplantation of tumor cells occur during sexual mating, sniffing, licking, or scratching of affected genitalia providing the major mode of transmission to the genital mucosa, nasal, and oral mucosa (Amaral et al., 2004). This neoplasm occurs predominantly as a tumor mass tissue in the female’s vaginal vestibule or at the glans bulb of the penis in males (Ganguly et al., 2016). In the current study, the lesions of TVT were restricted to the glans penis with no metastasis to any organs. The tumor mostly appeared as a friable, red, hemorrhagic, cauliflower-like mass and this finding is consistent with the reported by Ayala-Díaz et al. (2019) and Thangathurai et al. (2008).
The diagnosis of TVT was reinforced by histopathological investigations as we observed numerous, round to oval cells growing in rows within scanty fibrous stromal tissue. The tumor cells possessed slightly granular, vacuolated, and eosinophilic cytoplasm and large vesicular nuclei with prominent single nucleoli. Also, mitotic figures were plentiful. These results are in agreement with Ayala-Díaz et al. (2019), Fathi et al. (2018), Mascarenhas et al. (2017) and Behera et al. (2012). The present study confirms that the IV administration of vincristine sulfate (0.025 mg/kg, once a week for 2 weeks) was very effective in the complete remission of TVT in male dogs without any recurrence, and these results are in a harmony with previously published studies (Hiblu et al., 2019; Antonov, 2015; Özalp et al., 2012).
Vincristine sulfate is commonly used as an effective, safe chemotherapeutic drug for TVT cases worldwide. Setthawongsin et al. (2019) suggested a better response with vincristine sulfate alone in comparison to combined chemotherapy. According to Ganguly et al. (2016), IV administration of vincristine sulfate at a dose of 0.025 mg/kg for 15 days beyond the complete regression of the neoplastic mass is considered as the treatment of choice regardless of the duration of the disease, the tumor size, and the degree and the presence of metastasis. Vincristine sulfate has a specific effect in bacterial multiplication (Tella, 2004), the cell cycle during tumor regression, and induces apoptosis (Setthawongsin et al.,2019). This is supported by Gonzalez et al. (2000) who found that after treatment by vincristine sulfate, tumor cell proliferation ceased, apoptosis increased, leucocytes increased, and tumor parenchyma collapsed around intra-tumoral vessels in addition to increasing fibrosis. Furthermore, Bates and Eastman (2017) and Özalp et al. (2012) demonstrated that vincristine can disrupt microtubules formation via high-affinity binding to tubulin.
Normal b-mode grey ultrasound revealed a tool for evaluation of the normal genital tract of the male dog as well as became a tool and widely used in the dog’s clinical practice to differentiate between normal and abnormal testicular and prostatic lesion, but the color and spectral Doppler added novel information to the normal grey sonar that allows most doctors to get a more specific diagnosis (Jiang et al., 2011). The use of Doppler color and pulsed wave modes allow a very quick diagnosis as all examinations do not require any analgesic agents. The increase in vascular perfusion expressed by increasing blood flow rate and decreasing both Doppler indices with around neoplastic round cells that are present at the early stages of the pathologic process as TVT, supplies the professionals’ useful information on the specific origin and gives additional information which improves the andrological diagnostics technique in the dog. As most non –neoplastic tumors form nodules but do not report any changes in blood flow waveform velocities and also all necrotic areas typically do not show increased vascularity and no changes occur in the spectral graph of the known specific arteries of the penis (Günzel-Apel et al., 2001). The new blood vessels form a huge network around the tumor cells and at the tumor marginal side which leads to a linear sharp increase in the vascularization represented by a marked decrease in the Doppler indices as there was an inverse relationship between blood supply and Doppler indices (Hori et al., 2014). In agreement with another study (Horstman et al., 1992), the peak systolic velocity in the known specific artery elevated with the increase in neoplastic nodules size. Even though, several authors found that there was a relationship between testicular and penile tumor in the size and amount of the vascularity with blood flow waveforms to the testicle and the penis (Forster et al., 2017). Similar to our finding, Doppler workup of the penile vessels in human patients could be used as preoperative evaluation in addition to accurate and rapid early detection of penile affection as fibrosis (Jung et al., 2018). Additionally, the recent advent of ultrasonography by detection of blood supply movement is also expected to provide valuable information on penile pathological affection by evaluating the vascularization variations in the penis and could help in the early rapid treatment and much research is being performed into these possibilities (Belgrano et al., 2008). In male dogs, the most important Doppler indices expressed as resistive index or resistance index (RI) is used as a diagnostic tool for the many scrotal inflammatory disease detection and evaluation (Carrillo et al., 2012). Furthermore, some authors demonstrated that RI was elevated in dogs with pathological sperm counts. The RI is similar in value in many types of testicular tumors only along with the PI values and no reports about the penile tumors. However, no reports about evaluating the penile vascularization in the case of venereal tumors present on penile tissue, so the blood flow vascular patterns measured in this study are adjective elements for the specific diagnostic hypothesis generation (Kocako et al., 2007). Despite the diagnosis of tumors spreading in the testis, penis, and prepuce is mostly dependent upon histopathological evaluation, color and spectral wave Doppler ultrasound could actually increase the confidence for accurate diagnosis and should be considered therefore one of the safest and dependable lines of investigation for testicular and penile lesions in dogs. In the present study, the Doppler sonography showed a high flow resistance pattern, after treatment by vincristine which resembled that of venous thrombosis in dogs. In agreement with our results; the Doppler parameters findings of venous thrombosis reported that an absence of venous flow with a high resistive or resistance arterial waveform (Foshager et al., 1997; Fitzgerald et al., 1992) that means low blood flow to the specific organ due to increased resistance (resistive) index to the artery.
Conclusion
The study provides the readers with a data range to assessing penile health as well as pathological changes of vascular perfusion in the case of dogs suffering from TVT. Color and pulsed Doppler sonography are obviously improving andrological diagnostics in addition to its value for the diagnosis of other diseases and tumors of the male dog.
Acknowledgements
The authors thank Dr. Refaat Ragab, professor of pets’ clinic in the faculty of veterinary medicine, Cairo University.
Authors contribution
Elshymaa Ahmed Abdelnaby, Yara Sayed Abouelela and Noha Ali Elsayed Yasin designed the protocol, collected the samples, performed the practical work and drafted the manuscript. All authors reviewed and approved the last version of the manuscript.
Conflict of interest
There are no conflicts of interest to declare.
Data availability
Data sharing is not applicable to this article as no new data were created in this study
Funding
The authors ́ research included into this article did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References





