Advances in Animal and Veterinary Sciences
Research Article
Evaluation of Osteopontin Expression in Liver and Colon of Murine Schistosomaiasis mansoni Model: A Pathological and Immunohistochemical Study
Hend Mohamed Hussein1*, Nehal A Radwan2, Noha Abdelfattah Elleboudy1*, Reham Fathy Tash3, Youssef Shoukry3, Ahmed H Abdelazeem4,5, Shimaa Abdelraouf Elgohary2
1Department of Parasitology, Faculty of Medicine, Ain Shams University, Cairo 11591, Egypt; 2Department of Pathology, Faculty of Medicine, Ain Shams University, Cairo 11591, Egypt; 3Department of Anatomy, Faculty of Medicine, Ain Shams University, Cairo 11591, Egypt; 4Department of Medicinal Chemistry, Faculty of pharmacy, Beni-Suef University, Beni-Suef 62514, Egypt; 5Department of Pharmaceutical Sciences, College of Pharmacy, Riyadh Elm University, Riyadh 11681, Saudi Arabia.
Abstract | Osteopontin is a granulomatogenic chemokine considered to be an inflammatory progenitor suggested to participate in hepatopathology in schistosomiasis. The aim of the current study is to evaluate Osteopontin immunohistochemical expression in chronic murine Schistosoma mansoni infection through week 7 till week 23 in both liver and colon histopathology samples, correlating them to serum glutamic-oxaloacetic transaminase and glutamate pyruvate transaminase levels. The results revealed significant association between levels of SGOT and SGPT and percentage of liver inflammation (P =0 .037 and 0.002 respectively), also smaller and bigger sized granulomas were significantly linked to low level of SGPT (P = 0.005). A parallel histopathologic change over time was detected in both liver and colon specimens. The infected groups show acute granuloma formation associated with liver fibrosis from week 7 through week 23. Changes in the expression of Osteopontin was observed at week 7. Upregulated expression was strongly seen from week 11 to week 13. At week 13 to week 17 the fibrotic granulomas reveal many positive cells, from week 21 to week 23 the expression was weak and almost was returned to near normal levels. Intensity of the expression was positively significantly correlated with the degree of inflammation, granuloma size and the degree of fibrosis (P < 0.01). This study highlights the use of OPN as a potential diagnostic marker of liver injury and the probability of using an anti OPN in early chronicity stage to prevent schistosomal subsequent pathological changes.
Keywords | Schistosoma mansoni, Osteopontin, Granuloma, Liver fibrosis, Colon.
Received | October 14, 2020; Accepted | December 12, 2020; Published | June 15, 2021
*Correspondence | Hend Mohamed Hussein, Noha Abdelfattah Elleboudy, Department of Parasitology, Faculty of Medicine, Ain Shams University, Cairo 11591, Egypt; Email: D.hend_m@yahoo.com, drnoha_elleboudy@med.asu.edu.eg
Citation | Hussein HM, Radwan NA, Elleboudy NA, Tash RF, Shoukry Y, Abdelazeem AH, Elgohary SA (2021). Evaluation of osteopontin expression in liver and colon of murine schistosomaiasis mansoni model: a pathological and immunohistochemical study. Adv. Anim. Vet. Sci. 9(7): 1078-1086.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.7.1078.1086
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Hussein et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Schistosomiasis has been considered by the World Health Organization (WHO) as one of the neglected Infectious diseases (NIDs). Schistosomiasis is an important public health problem and presents an economic burden since a review of the disease burden estimated that more than 200 000 deaths per year in sub-Saharan Africa. There is approximately 779 million people are at risk of infection worldwide. The Global Health Estimates disease burden over the past decade from 1.7 million disability-adjusted life years (DALYs) to as many as 56 million DALYs, depending on the disease prevalence (Hotez et al., 2014;WHO, 2016; Naghavi, 2017).
Three major species of Schistosoma are endemic and leads to human disease; Schistosoma mansoni (S. mansoni) in Africa, the Middle East, and South America, Schistosoma japonicum (S. japonicum) common in Southeast Asia, and Schistosoma haematobium (S. haematobium) mainly in Africa and the Middle East (Gryseels et al., 2006).
In Egypt, although there is decline in prevalence rates of schistosomiasis mansoni but there are still several recent surveys that report it in different areas (Elmorshedy et al., 2020). Reports associate schistosomiasis mansoni with 20.8% of colonic cases (Sabry et al., 2015), and with 26.7% of hepatocellular carcinoma patients (Gad et al., 2011).
Osteopontin (OPN) as an extracellular matrix acidic- glycophosphoprotein expressed by several cell types binds to CD44to mediate many biological and immunological processes (Matsue et al., 2015; Cabiati et al., 2017). OPN can also indorse inflammation (Kelly et al., 2005; Naldini et al., 2006). It encourages tissue remodeling, upregulate fibrosis, and help in angiogenesis (El-Asrar et al., 2012).
Osteopontine was suggested to be a better marker to follow up the prognosis of hepatocellular carcinoma (HCC) in its early stages of development than alpha-fetoprotein (AFP) (Shang et al., 2012).
The aim of this study was to evaluate the dynamics of OPN expression in chronic murine Schistosoma mansoni infection through week 7 till week 23 in both liver and colon histopathology samples.
Material and methods
Experimental animals
Fifty wiss albino female CD-1 six weeks old mice with average weight of 20 g, were bred and maintained at Theodor Bilharz Research Institute, the experimental animal research unit of the Biological Supply Unit at, Giza, Egypt. Following the U.S. Public Health Service Policy on the Use of Laboratory Animals for animal experimentation and in accordance with the ethical committee of Faculty of medicine Ain Shams University animal care protocol. With; air-conditioned room at 21°C in a 12-h light/dark cycle, 50 % humidity, and free access to standard commercial pelleted diet and water. Another fifty mice same weight and age were used as a matching control.
Schistosoma mansoni infection
At Theodor Bilharz Research Institute, S. mansoni Egyptian strain cercariae were provided by the Malacology Lab of the Schistosomiasis Unit. Subcutaneous infection was performed using freshly shed 80 S. mansoni cercariae to each mouse through abdominal exposure in non-chlorinated water for 20 min. Confirmation of infection was by finding S. mansoni eggs in mice stool.
Study groups and assayed parameters
At 7, 9, 11, 13,15,17,19, 21 and 23 weeks after infection, 5 mice were randomly selected and sacrificed. Blood samples collected during sacrificing the mice. Liver and colon samples were collected for further studies. To obtain the schistosomes and worm count; portal perfusion was performed according to Pellegrino and Siqueira (1956). A control of matching weight and age were processed in the same way.
Biochemical assays: were used for glutamic-oxaloacetic transaminase (SGOT) or Aspartate transaminase (AST) and glutamate pyruvate transaminase (SGPT) or Alanine aminotransferase (ALT) assay by commercial assay kit (Spinreact, S. A. Ctra. Santa Coloma, Spain). (Young, 2001) by (UniCel® DxC 800 Synchron® Clinical System (Beckman Coulter GmbH). According to the manufacturer instructions.
To obtain the schistosomes and worm count; portal perfusion was performed according to Pellegrino and Siqueira (1956).
Histopathological study
Liver specimens were obtained, and the distal 3 cm of the colon was cut and washed with saline. They were fixed in 10% formalin and graded alcohol series were used to dehydrate the specimens. The specimens were then treated with xylene and embedded in paraffin blocks. the blocks were cut into 5-μm thick sections, and serial sections were placed on glass slides. Then according to standard procedures, the sections were stained with hematoxylin and eosin (H&E) and Masson trichrome (MT) according to Bancroft and Gamble (2008).
To evaluate and assess liver and large intestinal histopathological changes, two pathologists examined 10 different low-power fields of H&E and MT-stained sections (selected fields were in almost the same location) for each mouse (Chen et al., 2011). Histopathological score in these sections was determined according to Warren et al. (1975) & Ashour et al. (2015) with modifications. The degree of inflammation was scored from (0 to 3+). In addition, an ocular micrometer is used to measure the dimensions of non-confluent granulomas containing eggs in their centers at a magnification of 100× (Lichtenberg, 1962). Granuloma dimension = maximum width × maximum length. Mean granuloma dimension of each section equal the sum of all granuloma dimensions in each section divided on the number of granulomas in each section. Furthermore, each MT-stained section was examined at 100 × magnification to evaluate the degree of liver fibrosis. The degree of fibrosis was scored in each section based on (0 to 3+) (Warren et al., 1975).
Table 1: Descriptive statistics of parasitological parameters and liver function test
| Number | Median | Minimum | Maximum | Percentile | ||
| 25% | 75% | |||||
| SGOT IU/L | 60 | 321.5 | 172 | 453 | 248.5 | 388 |
| SGPT IU/L | 60 | 69.5 | 10 | 310 | 40 | 84 |
| Male worms count | 54 | 1.5 | 0 | 6 | 1 | 3 |
| Female worms count | 54 | 1 | 0 | 7 | 0 | 2.25 |
| Copula count | 54 | 3 | 0 | 4 | 2 |
3 |
Immunohistochemical study
Immunohistochemical (IHC) staining for OPN was conducted on sections 5µm thickness using an automated immunostainer (Roche Ventana, BenchMark ULTRA system) according to the manufacturer’s instructions primary antibody incubation against mouse monoclonal anti-Osteopontin antibody (1:200 dilution; clone sc-21742 Santa Cruz Biotechnology).
The immunoreactivity of OPN was evaluated and OPN expression was scored according to Ito et al. (2006) and Higashiyama et al. (2017) as follows: negative (0); weak or focal expression (1+); moderate expression with focal strong expression (2+); and strong expression (3+). OPN expression is detected in the cytoplasm of the granuloma macrophages (O’Regan et al., 2001).
Evaluation of OPN expression was done by two pathologists without any prior information about each specimen. When different grades were assigned, the final agreement was obtained after careful review of the slides by multiheaded microscope session for adjustment of the inter-observer controversy.
Statistical analysis
Statistical analysis for different variables was done by IBM SPSS statistics (V. 24.0, IBM Corp., USA, 2016) was used. For descriptive data analysis of quantitative variables, the median, minimum and maximum were used. For description of qualitative variables were presented as number and percentage. Chi-square test was used to compare qualitative variables. (Plackett, 1983) P value ≤ 0.05 is considered significant, while p value of <0.001: highly significant (HS).
Stacked bar graph were used to depict numeric values across multiple categorical variables (Der and Everitt, 2014).
RESULTS
Parasitological and biochemical parameters
Parasitological parameters and liver functions tests descriptive statistics along the studied weeks are shown in Table 1.
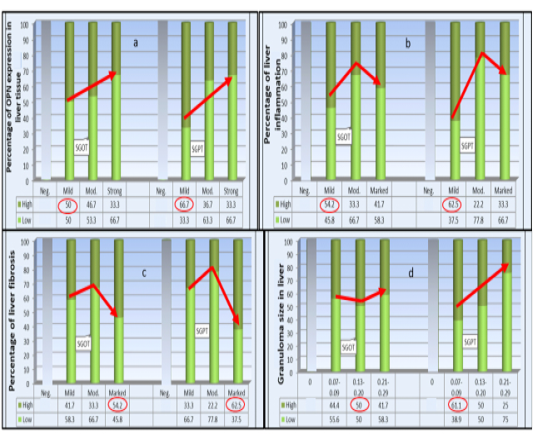
SGOT levels were categorized into low (172-322 IU/L) and (323-453 IU/L) as high. While SGPT levels were set to low (10-70 IU/L) and high (71-310 IU/L). Graph stacking of the biochemical results were presented in stacked bar chart (Plate 1), to study their changes in relation to liver OPN expression, fibrosis, inflammation, and granuloma size using Pearson Chi- square test. Liver OPN expression in relation to changes in both SGOP and SGPT levels showed inverse association in high levels and the reverse with their low values (Plate 1a) (P =0 .545 and 0.144 respectively).
Concerning the percentage of liver inflammation also an inverse association with high SGOT and SGPT levels (P =0 .037 and 0.002 respectively) was detected. As high levels of SGOT and SGPT were found among cases with mild inflammatory intensity (Plate 1b).
SGOT and SGPT serum levels show a. Non-significant inverse association with the degree of OPN expression -b. Significant inverse association with the percentage of liver inflammation -c. Significant association with liver fibrosis-d. Non-significant association SGOT with liver granuloma size while significant for SGPT by Pearson Chi-Square (P < 0.05). The highest percentage of all categories are circled.
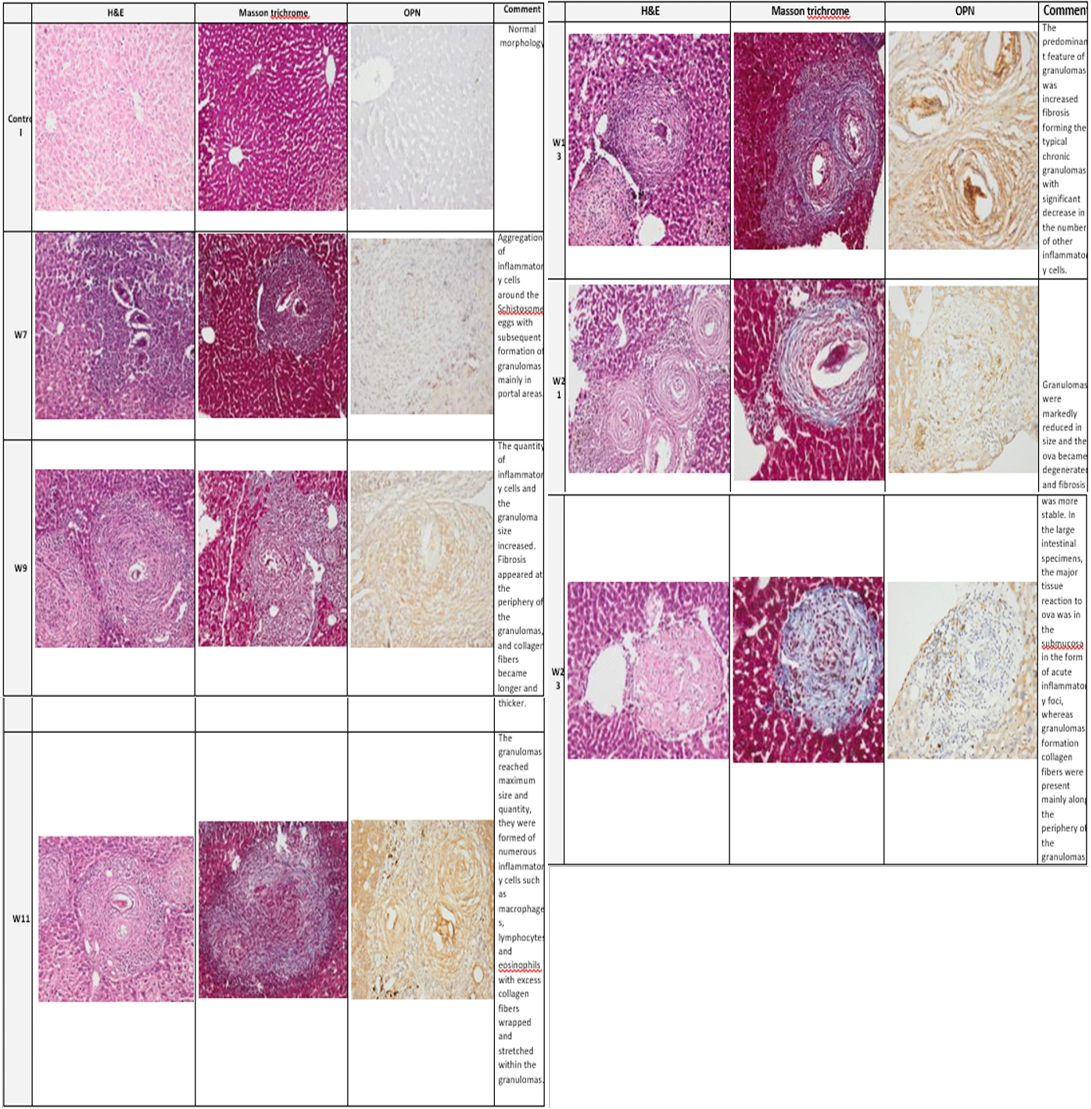
Plate (2): Photomicrograph of the mice liver
Photomicrograph of the mice liver showing the normal group (control) architecture while the infected group showed typical schistosomal hepatopathological characteristics with granuloma formation associated with subsequent fibrosis from week 7 through week 23. (H&E x100, Masson trichrome X 100, Anti Osteopontin Ab OPN X 100).
While stratification against liver fibrosis score was significant (P =0 .037) for SGOT and for SGPT (P=0.002) with highest serum levels among those with marked fibrosis. Finally, the difference in liver granuloma size was non- significantly associated with serum level of SGOT (P = 0.076) whereas for SGPT levels; got inverse significant link to the granulomas size and the reverse regarding its low level (P = 0.005).
Histopathological results
Typical schistosomal pathology was presented up on staining with both H&E and MT in both liver and colon samples starting from the 7th week till the 23rd expressed in (Plates 2 & 3) with similar changes over time with the percentage of fibrosis and the granuloma dimensions.
Immunocytochemical results
Highly significant adjoined variation of OPN intensity
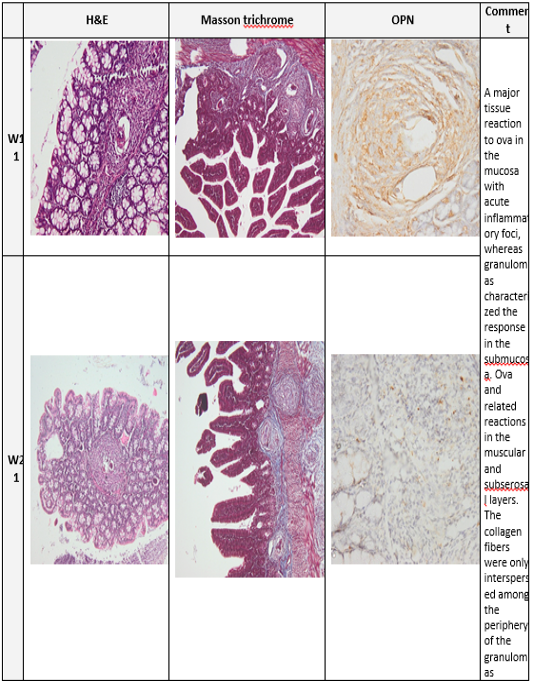
Plate (3): Photomicrograph of the mice colon
Photomicrograph of the mice colon of the infected group at week 11 and 21. (H&E x100, Masson trichrome X 100, Anti Osteopontin Ab OPN X 100)
with S. mansoni infection along the studied weeks of infection was detected by Pearson Chi-square test for liv er (150.000, P=0.0001) and colon (162.660, P=0.0001). Mostly no staining was demonstrated in the normal control group). At week 7 in the model group, the area of inflammatory cell infiltration shows wide distribution of positively stained cells, forming acute granulomas. Upregulated expression of OPN expression was strongly seen from week 11 to week 13.
Many compactly stained positive cells surround and infiltrate into the granulomas, are mainly accumulated in the fibrotic areas, and along the fibrous strands.
At week 13 to week17 the fibrotic granulomas reveal many positive cells which are distributed and dispersed mainly at its periphery of granulomas. However, from week 21 to week 23 OPN expression was weak and almost was returned to near normal levels as shown in (plates 2&3, right column).
Statistical analysis
Statistical analysis revealed that intensity of OPN expression was significantly associated with the degree of liver inflammation; The highest present of negative OPN intensity was found among no inflammatory cases; while the highest present of mild OPN intensity (n=12) was found among those with mild inflammatory cases, among those moderate OPN intensity (n=30); the highest percent was found among those mild and moderate inflammatory cases; while those strong OPN intensity (n=6); the highest percent was found among those moderate inflammatory cases (p<0.001, HS) (Plate 4-a).
As for the granuloma size the highest percent of cases with negative OPN intensity (n=12); was found among those without granuloma cases; while the highest present of mild OPN intensity the highest percent was found among those 0.07-0.09 granuloma size; among those moderate OPN intensity (n=30); the highest percent was found among those 0.21-0.29 granuloma size while those strong OPN intensity (n=6); the highest percent was found among those 0.21-0.29 granuloma size (p <0.001, HS) (Plate 4-b).
Finally, regarding the degree of fibrosis the highest percent of cases with negative OPN intensity (n=12); was found among those fibrosis score 0 cases; while the highest present of mild OPN intensity (n=12); was found among those marked fibrosis score; among those moderate (n=30); the highest percent was found among those moderate, and marked fibrosis score while those with strong OPN intensity (n=6); the highest percent was found among those marked fibrosis score (p<0.001, HS) (Plate 4-c). The association of OPN expression percentages in liver and colon samples were also significant where, highest percentage among liver samples (n=60) were with moderate expression whereas, in colon samples (n=59) the highest percentage with mild expression (Plate 4-d).
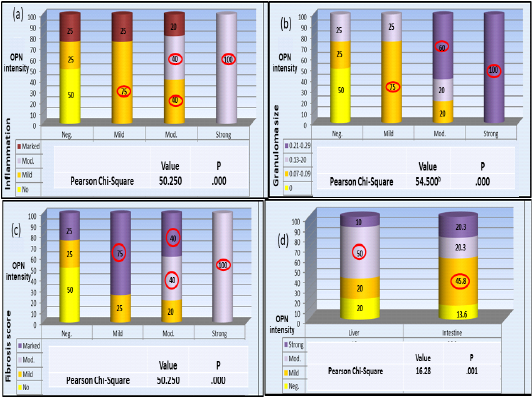
Plate (4): OPN percentage of expression in association with liver pathology variables a- inflammation b- Granuloma size c- Fibrosis score. d- Variation of OPN expression pattern between liver and colon samples.
The highest percentage of all categories are circled. Pearson Chi-square test in all results were significant P < 0.05
Discussion
OPN role in liver diseases was discussed in several studies focusing on its role as inflammatory progenitor and macrophage/neutrophils attractant. In hepatitis, hepatocellular and colorectal carcinomas special emphasis on its potentiality as a diagnostic and prognostic biomarker was mentioned (Agrawal et al., 2002; Rohde et al., 2007; Likui et al., 2010; Shang et al., 2012;Wen et al., 2016). OPN plays a leading role in granuloma formation and progression of fibrosis (Morimoto et al., 2004; Wang et al., 2014; Coombes et al., 2015). OPN can binds with receptors as CD44, αv integrin, vimentin, estrogen-related receptor alpha and MYD88 inducing infiltration, migration, invasion, and metastasis of cells (Boudjadi, et al., 2013; Wen et al., 2016).
Switching of the host response from Th1 to Th2-mediated response occurs around six to eight weeks post infection. With the stage of egg deposition, soluble egg antigen triggers the granulomatous inflammatory response (Colley et al., 2014). As schistosomal pathology does not affect liver cells (Lambertucci et al., 2014), so no changes in serum transaminases levels are expected in early cases (Sherwood et al., 2014). The studied enzymes showed significant association with the severity of fibrosis in agreement with Camacho-Lobato et al. (1998) and Leite et al. (2013). This could be attributed to the progress of fibrosis affecting liver function and leading to these changes. In this study levels of transaminases were significantly negatively related to both the level of liver inflammation and level of OPN expression in contrast to Tang et al. (2020) who detected increase in SGPT with liver inflammation along with increase OPN expression. That is may be credited to the difference in the etiological origin of the liver condition between both studies.
In the current study, typical colonic and hepatopathological changes were induced in S. mansoni-infected animals and Osteopontin immunohistochemical expression displayed a strong correlation with pathologic changes in the liver and colon. OPN levels were up regulated by schistosomiasis and positively correlated with the degree of fibrosis in the liver as detected by Chen et al. (2011) who studied week6 till week18. With peaking levels in week 10 then declining by week 18. This goes with the dynamics of OPN expression achieved from this study from week7 till week 13 where peaking occurred then declining happened. Pereira et al. (2015) also had similar results in OPN expression in week16 compared to week6 post infection. Science the aforementioned study was only limited to two readings and no variation in the degree of expression was studied, no available data could be compared to ours as we choose a longer course of infection leading to a decline in OPN expression overtime. When the state of immune inertia is reached as the period of late chronicity develops (Hams et al., 2013) and immunomodulation of the disease develops, OPN downregulation could be expected. The dynamics profile of OPN achieved suggests that it plays a role in early development of pathological changes then its role declines afterwards.
The significant association between high OPN expression and bigger granuloma size detected suggest that OPN expression is linked to Th1/Th2 response to granuloma formation as OPN production can attract inflammatory cells producing granulomas. In agreement with Morimoto et al. (2004) who stated that OPN can affect the granulomatous reaction in different model of granulomatous liver disease. Despite differences in some immunologic mechanisms and variation in size and consistency of granulomas in between S. mansoni and S.japonicum, Chen et al. (2011) reported a positive linear regression between OPN mRNA expression and granuloma size which intern accentuates the suggested role of OPN in schistosomal pathology.
A statistically highly significant variation in both liver and colon OPN intensity was found; the expression started in week7 and gradually increasing over time to peak in week 13 in liver and week 11 and 13 all together in colon. Afterwards decline in the degree of expression was recorded to reach its mild form by week 21 to 23. Finally, a highly significant variation in OPN intensity between liver and colonic tissue as moderate OPN intensity goes best with liver samples whereas mild in colonic samples. This difference may be attributed to multiple origin of OPN production highlighting the liver contribution to its production by Kupffer cells. Then again murine colonic granulomas and fibrosis were stated to be smaller as they are not downregulated as those of the liver granulomas (Cheever et al., 2002) so these data could explain why OPN expression varied between liver and colon though well detected in both samples.
Thus, our data emphasize the contribution of Osteopontin in the development of schistosomal colonic and hepatopathologic changes. And the expression profile obtained may open insights in using anti OPN during early stages of inflammation and granuloma formation to prevent the pathological damage.
Limitation of the study
A larger sample size may provide a more accurate results especially regarding laboratory results. The study could not eliminate the effect of fat in diet on the liver OPN expression.
CONCLUSION
The dynamics of OPN expression and its link to pathological changes in both liver and colon may add another piece of information to help in solving the puzzle of schistosomiasis impact proposing a new prognostic and therapeutic target. This study highlights the use of OPN as a potential diagnostic marker of liver injury and the probability of using an anti OPN in early chronicity stage to prevent schistosomal subsequent pathological changes.
Ethical consideration
The study was approved by the Research Ethics Committee, Faculty of Medicine, Ain Shams University. All the animal experiments were performed according to the Original article and national regulations for the Animal Ethics rules, Ain-Shams University, Cairo, Egypt.
acknowledgements
All thanks goes to TBRI Schistosome Biological Supply Center. Special thanks to Emeritus Prof. Soheir Sayed Mohammed Mahmoud.
Conflict of interest
The authors declare that they have no conflict of interest.
authors contribution
Authors HH and NE equally contributed in the experimental animal model as well as manuscript writing, revision and approval. NR, RT, YS and SE handled the pathology and immnunohistochemical part. AH managed the biochemical analysis.
REFERENCES