Advances in Animal and Veterinary Sciences
Research Article
Protective Effects of Thymus Vulgaris Oil Against CCL4-Mediated Hepatotoxicity, Oxidative Stress and Immunosuppression in Male Albino Rats
Gehad E. Elshopakey1, Engy F. Risha1*, Mohamed E. El-Boshy1,2, Osama A. Abdalla3, Mohamed F. Hamed4
1Department of Clinical Pathology, Faculty of Veterinary Medicine, Mansoura University, Mansoura, Egypt; 2Department of Laboratory Medicine, Faculty of Applied Medical Sciences, Umm, Al-Qura University Makka, Saudi Arabia; 3Department of Clinical Pathology, Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt; 4Department of Pathology, Faculty of Veterinary Medicine, Mansoura University, Mansoura, Egypt.
Abstract | A variety of alternative medicine is aromatherapy that uses essential oils for treating and preventing disease. The current study was applied to investigate the possible protective effects of Thymus vulgaris essential oil (TEO) against carbon tetrachloride (CCl4) toxicity. Forty-eight rats were equally allocated into six experimental groups and effectively treated for 4 weeks as follows: control group; the group treated with CCl4 (1ml/ kg b.w) CCl4 twice a week; the groups treated daily with low and high doses of TEO (2.5 and 5 mg/kg b.w) and the groups received CCl4 twice a week and treated daily with TEO at the two tested doses. At the end of the treatment period, blood and tissue samples were collected for estimation of hematological, biochemical, antioxidant, immunological parameters, and histopathological assessment, and immunohistochemistry for cleave caspase and PCNA in hepatic tissue. CCl4 significantly decreased all hematological parameters except neutrophils and toxic neutrophils counts were significantly increased. Moreover, CCl4 increased the liver enzymes level and hepatic oxidative stress parameters. Meanwhile, the antioxidants, respiratory burst, phagocytic, serum lysozyme, and bactericidal activities were significantly suppressed in CCl4-intoxicated rats. Also, CCl4 raised serum levels of TNF-α, IL-6, and diminished IL-10, IL-12. It also increased caspase 3 and PCNA. The treatment with T. vulgaris oil attenuated the toxic effect of CCl4 in all hematological parameters. It also reduced the hepatotoxic effect of CCl4, enhanced the antioxidant system, and improved the innate immune parameters and inflammatory markers to their normal level. Based on histopathological analysis T. vulgaris oil ameliorates hepatocellular necrosis induced by CCl4. Immunohistochemical assessment depicted lowered immunoreactivity against cleaved caspase-3 expression, significantly in comparison to CCl4 treated group. Meanwhile, significantly increases at PCNA Immunoreactivity which indicates hepatocellular regeneration in T. vulgaris oil. More pronounced improvement was noticed in the group received the high dose of TEO. Our results suggested that Thymus vulgaris essential oil has a potential antioxidant activity and many protective effects against liver toxicity induced by CCl4 and this protection was dose-dependent.
Keywords | Thymus vulgaris oil; CCl4; Hepatoprotective; Oxidative stress; Inflammation; Apoptosis
Received | April 23, 2021; Accepted | May 06, 2021; Published | June 15, 2021
*Correspondence | Gehad E Elshopakey, Department of Clinical Pathology, Faculty of Veterinary Medicine, Mansoura University, Mansoura, Egypt; Email: engyrisha@yahoo.com
Citation | Elshopakey GE, Risha EF, El-Boshy ME, Abdalla OA, Hamed MF (2021). Protective effects of thymus vulgaris oil against ccl4-mediated hepatotoxicity, oxidative stress and immunosuppression in male albino rats. Adv. Anim. Vet. Sci. 9(7): 1053-1063.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.7.1053.1063
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Risha et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Several toxins, alcohol, autoimmune diseases, intemperance, intemperance drugs, and metabolic disturbance can induce liver damage and hepatic fibrosis (Zhang et al., 2013). Carbon tetrachloride (CCl4) is one of the powerful lipid-soluble hepatotoxic agents. The toxicity of CCl4 is probably caused by its oxidation by cytochrome P-450 present in the endoplasmic reticulum to subsequently form trichloromethyl radical (CCl3). In the presence of oxygen, CCl3 is converted into an extra toxic form known as CCl3O2 (Ganie et al., 2011). Exposure to CCl4 caused metabolic activation of reactive oxygen species (ROS), like hydroxyl radicals, hydrogen peroxide, superoxide anion, and other free radicals that generated during several metabolic reactions (Kohen and Nyska, 2002). The reactive free radical products of CCl4 can induce peroxidative degeneration in the liver through reacting with polyunsaturated fatty acids and prompt lipid peroxidation in the biological membrane and cellular components (Al-Rasheed et al., 2014, Ozsahin et al., 2009). Moreover, CCl4 leads to significant impairment of calcium homeostasis through increasing calcium ion permeability from the plasma membrane and ultimately necrotic cell death. CCl4 also promotes liver fibrosis via rising tissue inhibitors of metalloproteinases (TIMP-1) and stimulating collagen synthesis and accumulation (Adesanoye and Farombi, 2010).
Nowadays, there is growing attention for using the dry powder sample and crude extracts of medicinal and aromatic plants for the development and preparation of food additives and alternative imitative medicine (Rota et al., 2008). Many Plants are considered precious sources for phytochemicals that have antioxidant properties and can prohibit tissue injuries caused by free radicals (Syed et al., 2014).
Essential oils (EOs) can be extracted from fresh plant flowers or leaves and widely consumed as aroma additives in food, cosmetics, and pharmaceuticals. Thyme (Thymus vulgaris) is widely cultivated as herbal medicine, spice, and tea and is considered one of the well-known plants of Lamiaceae family and (Domaracký et al., 2007). Thyme possesses different beneficial effects, including bronchopulmonary disorders, sedative, antispasmodic, gastroenteric, anthelmintic, antimicrobial, carminative, diaphoretic, and antioxidant activities (Rustaiyan et al., 2000, Miura et al., 2002). T. vulgaris essential oil (TEO) is an enriched source of aromatic bioactive components such as thymol and carvacrol, p-cymene, and γ-terpinene (Burt et al., 2005). Thymol and carvacrol are the fundamental phenolic components of thyme which have a considerable role as antimicrobial agents and antioxidant scavengers (Hoffman-Pennesi and Wu, 2010). The current study was designed to analyze the hepatoprotective, potential antioxidant, and immunomodulatory effects of TEO against CCl4 induced toxicity in male albino rats.
MATERIAL AND METHODS
Chemicals
Thymus vulgaris oil (Pale Yellow to colorless; specific gravity 0.915- 0.935), nitroblue tetrazolium (NBT), dimethyl sulfoxide (DMSO), and diphenyltetrazolium bromide (MTT) were obtained from Sigma Company (Sigma Aldrich Louis, MO, USA). As well, carbon tetrachloride (CCl4) was purchased from ADWIA Company (Cairo, Egypt).
Experimental Animals And Design
Adult male albino rats (n=48; 130-160 g) were obtained from laboratory Animals House (Ministry of Public health, Helwan, Egypt). During 14 days of acclimatization, the animals were supplied with a control diet and water ad libitum. Then, the animals were randomly assigned into six groups (n=8 per group). The groups were treated as follows; Control group, topically treated daily with 1ml/kg b.w deionized water. CCl4 group, treated with 1ml/ kg b.w CCl4 suspended in olive oil (1:1 v/v) twice a week (Abdalla et al., 2013). TEO2.5 and TEO5 groups were treated daily with T. vulgaris essential oil (TEO) at doses 2.5 and 5 mg/kg b.w, respectively. TEO2.5-CCl4 and TEO5-CCl4 groups received daily TEO (2.5 and 5 mg/kg b.w, respectively) with CCl4 twice a week at the same previous dose. All treatments were applied intragastrically using a stomach tube for continues 4 weeks. The experiment design and treatment doses are summarized in Figure 1.
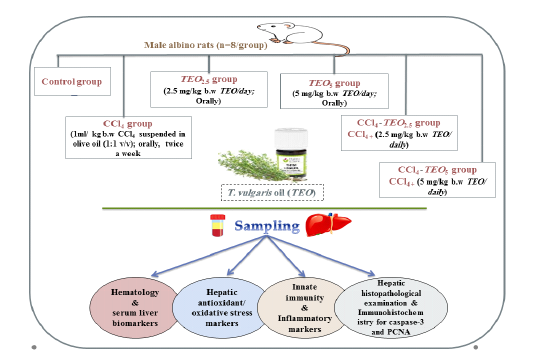
Figure 1: The experiment design and treatment doses used for TEO. CCl4; carbon tetrachloride, TEO; thymus vulgaris oil.
Samples Collection
At the end of the experiment, blood samples were collected and mixed with anticoagulant (EDTA) from the retro-orbital venous sinus of each animal for blood counting, respiratory burst, and phagocytic activity estimation. Another blood sample was also collected in a test tube and centrifuged at 3000 rpm (4°C) after being clotted for serum separation. The separated serum was stored at –80°C for further determination of biochemical and immunological parameters.
Rats were sacrificed and liver tissue specimens were collected. Part of liver tissue was homogenized in cold phosphate buffer saline (PBS), centrifuged and the supernatants were carefully picked up for evaluation of oxidative stress/antioxidants biomarkers (Fernandez-Botran et al., 2002).
Blood Cell Count
Complete blood count parameters were evaluated manually using a hemocytometer improved. The film was also prepared from each blood sample, fixed, and then stained with Gemisa stain for differential leukocytes’ count (Feldman et al., 2000).
Serum Liver Biomarkers
Following the stander protocol of each enclosed pamphlets, alanine and aspartate aminotransferases (ALT & AST; Randox Co., UK), alkaline phosphatase (ALP; Teco diagnostics Co., USA), total protein, and albumin (Stanbio Co., USA) were estimated spectrophotometrically (5010, Photometer, BM Co. Germany) in serum samples.
Antioxidant/ Oxidative Stress Markers
Using available commercial kits (Biodiagnostic, Egypt), the malondialdehyde (MDA), nitric oxide (NO), glutathione (GSH), glutathione peroxidase (GSH-Px), and superoxide dismutase (SOD), as well as, serum total antioxidant capacity (TAC) levels were evaluated spectrophotometrically (5010, Photometer, BM Co. Germany) in the hepatic homogenates.
Innate Immunity And Cytokines
Respiratory Burst Activity was estimated by using the nitroblue tetrazolium (NBT; Sigma-Aldrich, USA) reduction assay as previously reported (Berger and Slapnickov, 2003). The respiratory burst activity was measured at 630 nm as NBT reduction (O.D) using the microplate reader (Optica, Mikura Ltd, UK).
Phagocytic Activity was conducted following the previously modified method (Sawosz et al., 2009). Briefly, The blood cell suspension was incubated for 30 min at room temperature with Staphylococcus aureus (1X 108) cell suspended in phosphate-buffered saline (PBS) (1:1 ratio PBS: Bacteria). Later, the smears were prepared, fixed and stained with Giemsa stain. The bacteria containing cells (200 cells) were counted to calculate the phagocytic activity (%) as follows; Phagocytic activity= (Number of phagocytizing cells/ Total cells number) x 100.
Serum Lysozyme Activity was applied based on utilizing Micrococcus lysodeikticus (Sigma Chemical Co), with some modifications. The optical density (O.D) was measured at 540 nm and the concentrations were calculated using a lyophilized chicken egg-white lysozyme (Sigma, USA) calibration curve (Akinbi et al., 2000, Osama et al., 2014).
Serum Bactericidal Activity was assessed using diphenyltetrazolium bromide solution (MTT, 2 mg/ml) and E.coli bacterial suspension of (1 X 108), using the previously described methods with minor modifications (Kampen et al., 2005). The DMSO was used to dissolve the formed formazan and absorbance was taken at 560 nm (Optica, Mikura Ltd, UK) and expressed as an absorbance unit (O.D).
Serum Cytokines
Tumor necrosis factor-alpha (TNF-α), interleukins (IL-6, IL-10, and IL-12) levels were strictly evaluated using ELISA kits supplied by Invitrogen Co. (USA).
Histopathological Examination
The different hepatic tissues were fixed in formaldehyde (10%), and then paraffin-embedded sections were made. The different sections were stained with hematoxylin and eosin (H&E) stains. Slides were carefully examined for histopathological changes and lesion scoring (Table 4) (Gibson-Corley et al., 2013).
Immunohistochemical Estimation For Caspase-3 And Pcna
Sections were cut (4μm thick) to be mounted on saline-coated glass slides. Through a graded ethanol series, the sections were dehydrated after deparaffinization in xylol. Antigen retrieval was performed and endogenous peroxidase activity was blocked with H2O2 (3%). Later, tissue sections were incubated for 1 h at room temperature with primary antibodies against both caspase-3, Lab Vision™, Int’l: RUO, USA), monoclonal antibody (ready to use), 1:300, and PCNA (BioGenex, Milmont Drive Fremont, USA), rabbit monoclonal antibody at dilution 1:400. Secondary anti-rabbit antibodies were then added to different slides, and visualized by adding the 3,3’diaminobenzidine tetrahydrochloride liquid system (Dako). Counterstaining the sections with hematoxylin was applied before light microscopy examination (Olympus Corporation, Tokyo, Japan). Immunohistochemical quantifications were effectively conducted via Image J software (NIH, Bethesda, MD, USA).
Table 1: Hematological parameters (n = 8 per treatment) of albino male rats treated with thymus vulgaris Oil (TEO2.5 (2.5 mg/ kg b.w) and TEO5 (5 mg/ kg b.w)) and CCl4 for 4 weeks.
| Parameters | Groups | ||||||
| Control |
CCl4 |
TEO2.5 |
TEO5 |
TEO2.5+ CCl4 |
TEO5 +CCl4 |
|
|
|
RBCs (106/µL) |
8.01 ± 0.46a |
4.72 ± 0.41c |
8.22 ± 0.21a |
8.69 ± 0.36a |
5.18 ± 0.42c |
6.83 ± 0.57b |
|
| Hb (gm/dl) |
14.20 ± 0.83a |
8.06 ± 0.43c |
14.51 ± 0.64a |
14.71 ± 0.88a |
8.82 ± 0.56c |
10.63 ± 0.73b |
|
| PCV (%) |
47.40 ± 0.93a |
37.60 ± 1.21c |
49.71 ± 1.74a |
49.14 ± 1.46a |
38.25 ± 1.41c |
40.99 ± 1.68b |
|
| MCV (fl) |
57.27 ± 1.07b |
81.18 ± 1.99a |
59.02 ± 1.26b |
55.45 ± 1.27b |
73.79 ± 1.28a |
55.81 ± 1.31b |
|
| MCH ( pg) |
17.80 ± 0.20a |
17.20 ± 0.58a |
17.51 ± 0.44a |
16.86 ± 0.45a |
17.01 ± 0.59a |
15.53 ± 0.31a |
|
| MCHC (%) |
31.24 ± 1.39a |
21.40 ± 1.65b |
29.8 ± 1.32a |
31.06 ± 1.15a |
23.01 ± 1.26b |
26.72 ± 1.49a |
|
|
WBCs (103/µL) |
14.26 ± 0.82a |
8.40 ± 0.59c |
14.42 ± 1.52a |
15.82 ± 1.61a |
8.59 ± 1.58c |
10.69 ± 1.75b |
|
|
Lymphocyte (103/µL) |
12.01 ± 1.03a |
5.36 ± 0.79c |
12.72 ± 1.16a |
12.84 ± 1.29a |
5.84 ± 1.03c |
8.62 ± 0.91b |
|
|
Neutrophil (103/µL) |
2.16 ± 0.14b |
3.79 ± 0.21a |
2.25 ± 0.21b |
3.07 ± 0.25ab |
3.53 ± 0.39a |
2.18 ± 0.26b |
|
|
Toxic Neutrophil (103/µL) |
0.00 ± 0.00b |
0.24 ± 0.02a |
0.00 ± 0.00b |
0.00 ± 0.00b |
0.25 ± 0.02a |
0.16 ± 0.04ab |
|
Data were represented as Mean ± SEM. values within the same row with different superscripts superscript with different letters are significantly different (p < 0.05).
Table 2: Serum biochemical parameters (n = 8 per treatment) of albino male rats treated with thymus vulgaris Oil (TEO2.5 (2.5 mg/ kg b.w) and TEO5 (5 mg/ kg b.w)) and CCl4 for 4 weeks.
| Parameters | Groups | |||||||||||
| Control |
CCl4 |
TEO2.5 |
TEO5 |
TEO2.5+ CCl4 |
TEO5 +CCl4 |
|||||||
| ALT (U/L) |
35.07 ± 0.91c |
95.68 ± 2.03a |
34.62 ± 0.99c |
32.96 ± 1.19c |
89.49 ± 1.14a |
71.28 ± 1.38b |
|
|||||
| AST (U/L) |
56.31 ± 1.25c |
103.12 ±1.58a |
59.88 ± 2.34c |
53.93 ± 2.65c |
97.34 ± 2.33a |
79.07 ± 2.14b |
|
|||||
| ALP (U/L) |
183.9 ± 1.22c |
209.4 ± 1.5a |
186.4 ± 2.11c |
183.5 ±2.82c |
201.9 ± 1.59a |
195.1 ± 0.98b |
|
|||||
| Total Protein (g/dl) |
7.01 ± 0.32b |
4.95 ± 0.30c |
7.53 ± 0.61b |
8.72 ± 0.41a |
4.81 ± 0.42c |
6.13 ± 0.39b |
|
|||||
| Albumin (g/dl) |
3.87 ± 0.35a |
2.01 ± 0.18c |
3.44 ± 0.21a |
3.48 ± 0.25a |
2.13 ± 0.31c |
2.95 ± 0.27b |
|
|||||
| Globulin(g/dl) |
3.14 ± 0.43c |
2.94 ± 0.45c |
4.05 ± 0.58cb |
5.23 ± 0.61a |
2.53 ± 0.47cd |
3.18 ± 0.28c |
|
|||||
| A/G ratio |
1.23 ± 0.51a |
0.68 ± 0.33b |
0.87 ± 0.20ab |
0.66 ± 0.25b |
0.84 ± 0.17ab |
0.93 ± 0.20ab |
|
|||||
Data were represented as Mean ± SEM. values within the same row with different superscripts superscript with different letters are significantly different (p < 0.05).
Statistical Analysis
All data were analyzed using ANOVA/ Duncan multiple comparison tests except lesion scoring and immunohistochemistry was performed using Tukey/posthoc test (SPSS software program; version 20, USA); and expressed as mean ± stander errors of the mean (SEM).
RESULTS
Effects Of Teo And Ccl4 On Blood Count
Red blood cell count was not affected in TEO-treated groups compared with the control one. Meanwhile, RBCs count, Hb, PCV, TLC, and lymphocytic count were significantly decreased in the CCl4 group relative to control rats (p< 0.05). The treatment of the CCl4 group with a high dose of TEO (5 mg/kg b.w) significantly elevated the reduction in RBCs count, Hb, PCV, TLC, and lymphocytic count in contrast to the CCl4–intoxicated group (p< 0.05).
CCl4 treated rats showed higher MCV and lower MCHC than that of the control (p< 0.05). Meanwhile, the treated CCl4 group with TEO (5 mg/kg b.w) could correct the change in MCV and MCHC caused by CCl4. Both neutrophils and toxic neutrophils were significantly raised in CCl4 and TEO2.5-CCl4 groups unlike the other groups (p< 0.05) (Table 1).
Effects of Teo and Ccl4 on Liver Biomarkers
The CCl4 group represented significant hepatotoxicity demonstrated by higher activities of ALT, AST, and ALP compared to the control group (p< 0.05). The TEO-CCl4 treated group (5 mg/kg b.w) exhibited the ability of TEO to modify the CCl4 hepatotoxicity by decreasing ALT, AST, and ALP serum levels concerning CCl4 exposed group (p< 0.05) (Table 2). Proteinogram assay showed a significant reduction in serum total protein, albumin and albumin-globulin ratio in CCl4 exposed group compared with the control group (p< 0.05). TEO2.5 and TEO5 groups showed a significant elevation in total protein, globulin level, and reduction in the albumin-globulin ratio in contrast to the control rats (p< 0.05). Furthermore, the total
Table 3: Antioxidant and oxidative stress parameters (n = 8 per treatment) of albino male rats treated with thymus vulgaris Oil (TEO2.5 (2.5 mg/ kg b.w) and TEO5 (5 mg/ kg b.w)) and CCl4 for 4 weeks.
| Parameters | Groups | ||||||
| Control |
CCl4 |
TEO2.5 |
TEO5 |
TEO2.5+ CCl4 |
TEO5 +CCl4 |
|
|
| MDA (nmol/g liver tissue) |
49.22 ± 3.85c |
80.81 ± 2.31a |
50.22 ± 3.45c |
48.33 ± 2.92c |
76.54 ± 3.49a |
60.31 ± 2.56b |
|
| NO (µmol/l) |
18.85 ± 0.41c |
31.79 ± 1.01a |
18.57 ± 1.01c |
17.91 ± 0.97c |
31.25 ± 1.12a |
25.94 ± 0.89b |
|
| SOD (U/g liver tissue) |
288.1 ± 4.93b |
242.3± 4.88d |
292.8 ±4.85b |
306.5 ± 4.62a |
259.5 ± 5.14cd |
266.4 ± 5.52c |
|
|
GPX (U/g liver tissue) |
334.3 ± 2.08a |
321.1 ± 2.05c |
334.8 ± 3.87a |
339.1 ± 3.63a |
322.9 ± 3.69c |
328.5 ± 2.28b |
|
| GSH (mmol/g liver tissue) |
7.65 ± 0.41b |
2.87 ± 0.31d |
6.97 ± 0.29b |
8.73 ± 0.43a |
3.02 ± 0.39d |
5.13 ± 0.47c |
|
| TAC (mM/l) |
0.34 ± 0.019b |
0.12 ± 0.017d |
0.36 ± 0.031b |
0.45 ± 0.036a |
0.15 ± 0.028d |
0.28 ± 0.027c |
|
Data were represented as Mean ± SEM. values within the same row with different superscripts superscript with different letters are significantly different (p < 0.05).
Table 4: Lesion scoring system for the hepatic tissues
| Organ |
Score |
Histopathological Description |
|
Inflammation Score |
0 | Normal tissue architecture |
| 1 | Mild lobular inflammation (<10% of liver parenchyma)/mild portal inflammation (<1/3 of portal tracts) | |
| 2 | Moderate lobular inflammation (10–50% of liver parenchyma)/moderate portal inflammation (approximately 50% of portal tracks) | |
| 3 | Severe lobular inflammation (>50% of liver parenchyma)/severe portal inflammation (>2/3 of portal tracts). | |
|
Necrosis Score |
0 | Normal tissue architecture |
| 1 | <10% necrosis of liver parenchyma | |
| 2 | 10–25% necrosis of liver parenchyma | |
| 3 | >25% necrosis of liver parenchyma |
protein and albumin levels were significantly increased in TEO5-CCl4 group unlike the CC14-treated group (p < 0.05) (Table 2).
Effects of Teo And Ccl4 on Antioxidant/Oxidative Stress Markers
In this work, hepatic MDA and NO levels were significantly increased in CCl4 treated rats relative to the control (p< 0.05). Treatment with TEO (5 mg/kg b.w) inhibited the oxidative stress caused by CCl4 intoxication and lead to a significant reduction in MDA and NO levels in relation to CCl4 exposed group (p< 0.05).
TEO administration motivated a remarkable elevation in the activity of SOD as well as, GSH and TAC levels when compared to the control group (p< 0.05). The hepatic activity of SOD, GSH, GSH-Px, and serum TAC were significantly reduced in CCl4 and TEO2.5-CCl4 groups unlike the control rats (p< 0.05). Whereas, TEO5 treatment modified the reduction in antioxidant biomarkers caused by CCl4 intoxication as displayed in the Table (3).
Effects Of Teo And Ccl4 On Immune Parameters And Cytokines
The treatment of rats with TEO enhanced the innate immunity through elevation of respiratory burst and phagocytic activities relative to the control group (p< 0.05) (Fig. 2A & B). Meanwhile, CC14 intoxicated rats revealed a significant decrease in the respiratory burst, phagocytic, serum lysozyme, and bactericidal activities unlike the control rats (p< 0.05). The CCl4 group treated with the higher dose of TEO (5 mg/ kg) improved the respiratory burst, phagocytic, serum lysozyme, and bactericidal activities to their normal level (p< 0.05) (Figure 2).
The elevation of serum TNF-α and IL-6 levels reflect the liver injury as showed in CCl4 and TEO2.5-CCl4 groups (p< 0.05). Whereas, the treatment of CC14 intoxicated rats with TEO (5mg/ kg b.w) showed significantly reduced TNF-α and IL-6 levels unlike the CCl4 group (p< 0.05) (Fig. 3A& B). Additionally, cytokines (IL-10 and IL-12) can adjust the inflammatory response and relieve hepatotoxicity. The serum levels of IL-10 and IL-12 were significantly reduced in the CC14 group unlike the control one (p< 0.05). The rats that received TEO at a dose of 5mg/ kg b.w showed significant elevation in IL-10 and IL-12 levels compared to the control one (p< 0.05). As well, the treatment of CC14 with TEO (5mg/ kg b.w) significantly increased the IL-10 and IL-12 levels when compared with CCl4 exposed group (Fig. 3C& D).

Figure 2: Respiratory burst (A) phagocytic (B) serum lysozyme (C) and bactericidal activities (D) of albino male rats treated with thymus vulgaris oil (TEO2.5 (2.5 mg/ kg b.w) and TEO5 (5 mg/ kg b.w)) and CCl4 for 4 weeks. Data are expressed as Mean ± SEM. The different letters indicate significant difference (P < 0.05) between treated groups.
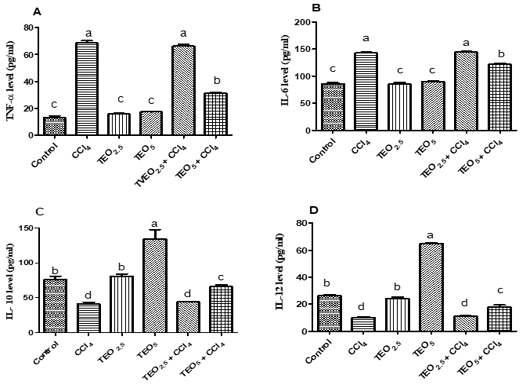
Figure 3: Serum cytokines levels; TNF-α (A), IL-6 (B), IL-10 (C) and IL-12 (D) of albino male rats treated with thymus vulgaris oil (TEO2.5 (2.5 mg/ kg b.w) and TEO5 (5 mg/ kg b.w)) and CCl4 for 4 weeks. Data are expressed as Mean ± SEM. The different letters indicate significant difference (P < 0.05) between treated groups.
Effects of Teo And Ccl4 On Histopathological/ Immunohistochemical Alterations
Histopathological examination displayed normal hepatocytes with normal hepatic architecture in control, TEO2.5, and TEO5 groups (Fig. 4A, B & C). Meanwhile, the CC14 group displayed vacuolation and coagulative
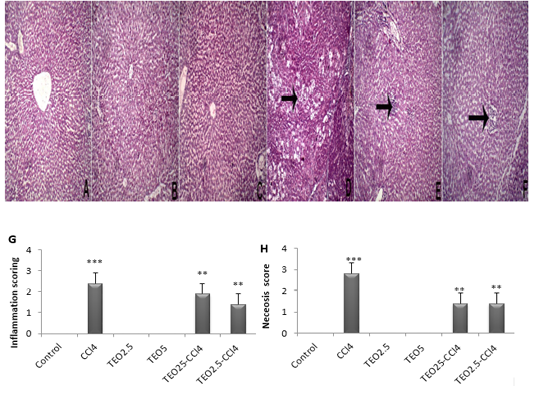
Figure 4: Hematoxylin and eosin staining of liver sections from rats. (A) Control, (B) TEO2.5, (C) TEO5, ( D) CCl4, (E) TEO2.5-CCl4 , (F) TEO5-CCl4 groups, (G) inflammation and (H) necrosis scoring for 4 weeks. Data are presented as mean ± standard error (***p value < 0.001).
necrosis of hepatocytes dispersed allover hepatic lobules (arrow) with intralobular fibroblastic proliferation (Fig. 4D). TEO2.5-CCl4 groups showed normal hepatic with intralobular round cells infiltration (Fig. 4E). As well, TEO5-CCl4 groups presented normal hepatic with round cell infiltration at the portal area (Fig. 3F).
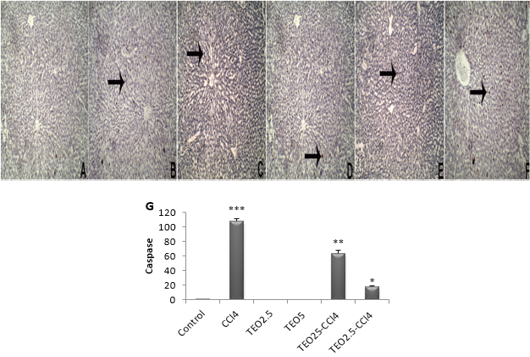
Figure 5: The immunohistochemical expression and quantitation of caspase 3 in the liver. (A) Control, (B) TEO2.5, (C) TEO5, (D) CCl4, (E) TEO2.5-CCl4, (F) TEO5-CCl4 groups and (G) scoring for 4 weeks. Data are expressed as Mean ± SEM. Data are presented as mean ± standard error (***p value < 0.001).
Apoptosis is programmed cell death of hepatocellular occurring along with necrosis. Accordingly, we used caspase-3-immunostaining to predict whether T. vulgaris oil treatments can spare hepatocytes from apoptosis alongside necrosis induced by CCL4. Caspase-3-immunostaining of apoptosis exhibited hepatocytes with strong positive staining in CCl4-intoxicated liver sections (Fig 5), as confirmed by caspase-3 quantification (Fig. 5G). Our results are pronouncedly boosted by immunohistochemistry against PCNA, which reflects the extent of hepatocytes proliferation, where there is a marked expression of PCNA at T. vulgaris oil treatment in comparison to CCl4 (Fig. 6).
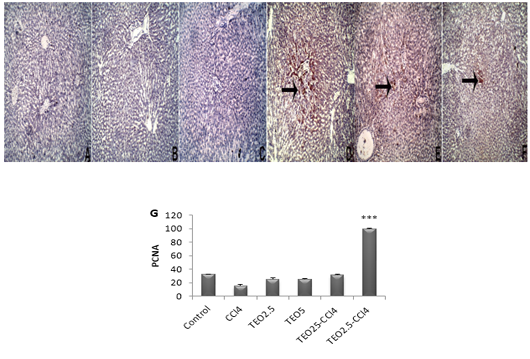
Figure 6: The immunohistochemical expression and quantitation of PCNA in the liver. (A) Control, (B) TEO2.5, (C) TEO5, ( D) CCl4, (E) TEO2.5-CCl4, (F) TEO5-CCl4 groups and (G) scoring for 4 weeks. Data are expressed as Mean ± SEM. Data are presented as mean ± standard error (***p value < 0.001).
DISCUSSION
T. vulgaris have several beneficial effects including antifungal, antimicrobial, carminative, antiseptic, and antioxidant activities. Phenolic compounds of its essential oils like thymol and carvacrol was noticed to have antioxidant and hepatoprotective potency (Baranauskienė et al., 2003). As well another study represented by Fachini-Queiroz et al. (2012) clarified that thymol and carvacrol of TEO have a modulatory effect on the inflammatory response. This study estimated the protective effects of TEO against CCl4 intoxicated rats. CCl4 is a known hepatotoxic substance which metabolized by cytochrome P-450 enzyme into trichloromethyl peroxide and chloroform (Avasarala et al., 2006). The free radical production conquers the antioxidant system of the liver and leads to oxidative destruction of cell membranes and stimulates the immune cells to liberate inflammatory mediators such as cyclooxygenase enzyme, interleukins, and cytokines which ultimately lead to leukocytes infiltration and damaged liver (Ferguson et al., 2004).
As displayed in our results, CCl4-treated rats displayed a considerable reduction in RBCs count, Hb content, and PCV which could be referred to as the disturbing hematopoiesis, erythrocytes destruction, reducing the erythrocyte formation rate as erythropoiesis is controlled by erythropoietin hormone which is primarily liberated by the kidney and liver (Meral and Kanter, 2003). On the other hand, CCl4 intoxication induced macrocytic hypochromic anemia as CCl4 increase the lipid peroxidation, proteins degradation in the cell membrane, alterations of membrane-bound enzymes, and also increase osmotic fragility of erythrocyte (Makni et al., 2012). Treating the rats with TEO (5 mg/kg b.w) modulates the toxic effect of CCl4 on erythrocyte as thymol prohibited the lipid peroxidation and able to scavenge the free radicals, subsequently, it could diminutive the hemolytic effect generated by CCl4 administration (Alam et al., 1999).
Leukogram in CCl4 exposed group showed a significant decrease in total leukocytes, lymphocytes, neutrophils, and increase in toxic neutrophils numbers which related to increased synthesis and release of TGF-β1 and other inflammatory biomarkers after CCl4 toxicity which suppress the immune responses (Guo et al., 2000). Furthermore, the neutrophilia is attributed to the capability of CCl4 enhanced production of Interleukin- 1β, and TNF-α from kupffer cell that stimulates other inflammatory mediators as granulocyte macrophage colony stimulating factor (GM-CSF) that accountable to differentiation and maturation of neutrophils (Wills and Asha, 2007). Elevation of toxic neutrophils may be imputed to the free radicals liberation during CCl4 metabolism which had an adverse effect on circulating white blood cell membranes and its content and could also induce several ultra-structural abnormalities in both cytoplasm and nucleus of leukocytes (Sinha et al., 2006). Treating CCl4 intoxicated rats with a high dose of TEO significantly elevated total leukocytes and lymphocyte count which could be correlated to the known anti-inflammatory effects of thymol (Chan et al., 2005).
The CCl4 group showed significantly higher serum activities of ALT, AST, and ALP as CCl4 cause an increase in cell membrane permeability and motivate hepatic cell damage resulted in the release of liver enzymes into the blood (Mir et al., 2010). The treatment of intoxicated rats with TEO (5 mg/kg b.w) significantly reduced ALT, AST, and ALP serum activities. This referred to the antioxidant power and free radical scavenging ability of thymol and carvacrol (Hazzit et al., 2006).
The serum total protein and albumin levels represented a highly significant decrease in CCl4 treated rats. These results may be due to the impaired liver function and hepatic cell damage caused by CCl4 (Kazeem et al., 2011). The dropping in the A/G ratio is imputed to the decrease in albumin which cannot be compensated by unchanged serum globulin. Thymol and carvacrol could increase the absorption of protein and globulin in the lower intestine which leads to their concentrations in serum (Rahimi et al., 2011). This also has been observed in the TEO5-CCl4 group which showed significant elevation in proteingram, unlike CCl4 intoxicated group due to its antioxidant and hepatoprotective efficacy (Hazzit et al., 2006).
In our work, hepatic MDA and NO levels were significantly higher in the CCl4 group. The treatment of rats with TEO (5 mg/kg b.w) significantly decreased the elevation in MDA and NO levels caused by CCl4 toxicity. This correlated to the protective, antioxidant, and free radical scavenging roles of thyme oil against CCl4 mediated liver injury (Ložienė et al., 2007). The antioxidants play an important role in the detoxification process of various toxic chemicals and xenobiotics (Olakunle Ajiboye, 2010). For example, GSH can promptly scavenge free radicals to conserve the biological systems from oxidative hurt (El-Sayed et al., 2015). As well, superoxide dismutase (SOD) can catalyze superoxide radicals into molecular oxygen and hydrogen peroxide (Coballase-Urrutia et al., 2011). As presented in the CCl4 group, the hepatic SOD, GSH-Px, GSH, and serum TAC levels were significantly exhausted due to the free radical production in the case of CCl4 intoxication (Cuciureanu et al., 2009). When we treated CC14 intoxicated rats with TEO (5 mg/kg b.w), a significant elevation in hepatic antioxidants was noticed. Moreover, the rats received TEO (5 mg/kg b.w) alone showed a remarkable increase in SOD and GSH activities, also in serum TAC level. These are assigned to the antioxidant properties and free radical scavenging of thymol and carvacrol (El-Nekeety et al., 2011).
In our results, CCl4 reduced the respiratory burst activity via inactivation of NADPH oxidase enzyme (Bishayi et al., 2002). NADPH oxidase enzyme present on phagocytic cell membranes which converted molecular oxygen into superoxide anion (O2−) that reduced redox dye NBT during a process known as the respiratory burst (Dügenci et al., 2003). TEO could stimulate the respiratory burst activity by an indirect method based on the reduction of Fe3+ to Fe2+ in a process known as ferric reducing antioxidant power which has been clarified by Miguel (2010). CCl4 also suppresses phagocytic activity through the release of numerous free radicals that adversely affect the leukocytes, or through stimulation of TGF-β1 production which down-regulate several immune responses (Sinha et al., 2006). The treated normal or intoxicated rats with TEO (5 mg/kg b.w) showed higher phagocytic activity as it was previously reported that carvacrol able to stimulate human natural killer cell and monocytes activities (Chan et al., 2005). As well, Ran et al. (2016) reported that Zebrafish fed 200 and 400 mg/kg of Next Enhance 150 (Essential oil product contain an equal volume of thymol and carvacrol) showed significantly higher respiratory burst and phagocytic activities of head kidney macrophages.
Lysozyme has an important role in innate immunity. It attacks the bacterial cell membrane and hydrolysis of N-acetylglucosamine and N-acetylmuramic acid causing lysis of bacterial cell wall (Bowden et al., 2004). CCl4 administration significantly reduces serum lysozyme level via suppression of macrophages maturation that produces lysozyme (Bishayi et al., 2002). Both TEO5 treated group’s revealed a significant elevation in lysozyme level. Miguel (2010) reasoned this to the ability of TEO to stimulate macrophages activity to produce lysozyme. In the present work, CCl4 treated rats showed significantly lower bactericidal activity. TEO (5 mg/kg b.w) can correct the reduction in bactericidal activity caused by CCl4 toxicity. This may be attributed to the ability of TEO to stimulate the immune cell phagocytic and respiratory burst activities and also elevated the serum lysozyme activity which has been confirmed in our results.
The exposure to CCl4 was reported to activate NF-κB with subsequent enhancing production of proinflammatory cytokines as TNF-α and IL-1β which are considered major hepatotoxicity mediators (Geier et al., 2002). In this experiment, CCl4 significantly elevated TNF-α and IL-6 levels and suppresses IL-10 and IL-12 secretion. This imputed to the CCl4 ability to stimulate the synthesis of TGF-β1 which interferes the macrophages activity to produce IL-12 and limits the main cellular immune responses (Guo et al., 2000). Treatment with TEO (5mg/ kg b.w) significantly decreased TNF-α and IL-6 but increase IL-10 and IL-12 serum levels. Also, Ocaña and Reglero (2012) reported that thyme extracts down-regulate TNF-α, IL-6, and IL-1B, beside an up-regulation of the IL-10 expression level of human macrophages. Chan et al. (2005) also revealed that the thymol has anti-inflammatory effects and it could stimulate the production of IL-12 from the immune cells.
One of the master apoptotic pathways is the mitochondrial apoptotic pathway (Tischner et al., 2010). Cytochrome C could activate caspase-3 to start cell apoptosis (Cusack et al., 2013). In this study, CCl4-generated hepatic apoptosis and the treatment with TEO could alleviate the apoptotic injury. The exposure of hepatocytes to hepatotoxic substances increased ROS production and promoted mitochondrial damage that subsequently, elevated the circulating levels of cytochrome C and caspase-3 as previously reported in CCl4-induced acute liver injury (Wu et al., 2016). The density of positive cells immunostained with PCNA antibody in the liver section was used as an indicator to evaluate the possible roles of TEO to stimulate hepatic regeneration. Our results strongly exhibited that TEO administration contributes to faster recovery following CCl4-mediated hepatic damage through promoting endogenous regeneration and suppressing apoptotic cell death (Zhu et al., 2013).
CONCLUSION
Overall, this study showed that TEO has efficient hepatoprotective effects in a dose-dependent manner. Besides, it has potent antioxidant, anti-inflammatory, and immunostimulant power that could minimize the CCl4-hepatotoxic effects. From the current study, it is suggested to use TEO as a future natural product for counteracting hepatic intoxication.
FUNDING
This research received no external funding.
CONFLICT OF INTEREST
The authors declare no conflict of interests
ACKNOWLEDGMENTS
All authors are very grateful to all members of the Clinical Pathology Department for their encouragement and support during this study.
authors contribution
G.E.E.; methodology, formal analysis, data curation,writing original draft, review and editing. E.R.., M.E.E., and O.A.A.; conceptualization, validation, visualization, and supervision. M.F.H.; histopathological & immunohistopathological examination, writing and revise the data of histopathology & immunohistopathology. E.R..; prepared the manuscript for publication. All authors read and approved the final manuscript.
REFERENCES





