Advances in Animal and Veterinary Sciences
Research Article
Pathogenicity and Immunogenicity of Attenuated Fowl Adenovirus from Chicken Embryo Liver Cells in Commercial Broiler Chickens
Norfitriah Mohamed Sohaimi1*, Mohd Hair Bejo1, Abdul Rahman Omar1, Aini Ideris1, Nurulfiza Mat Isa2
1Faculty of Veterinary Medicine, Universiti Putra Malaysia,Serdang, Selangor, Malaysia; 2Faculty of Biotechnology and Biomolecular Sciences, Universiti Putra Malaysia, Serdang, Selangor, Malaysia.
Abstract | Fowl adenovirus (FAdV) infection is a major viral threat to poultry industry worldwide. The virus cause Inclusion body hepatitis (IBH) outbreaks with serious economic losses and it demands an effective prevention and control measures of the disease. The objectives of this study were to determine pathogenicity and immunogenicity of live attenuated FAdV isolate (UPM1137) in commercial broiler chickens. The FAdV isolate was propagated into primary chicken embryo liver (CEL) cells for 35th passages (UPM1137CEL35) and induced several molecular changes in hexon and fiber genes at capsid region of viral protein. Sixty-four 1 day-old commercial broiler chickens were used and divided into three groups, 20 chicks in the groups A and B, and 24 chicks in the group C. The isolate with titer of 106.7TCID/ml was inoculated (0.5mL) into one-day-old chicks either via oral (Group A) or intraperitoneal (Group B) route. Control (Group C) was included and remained uninoculated. Samples of serum, liver and gizzard were collected in the chickens at day 0 post inoculation (pi) in the Control group and at days 3, 7, 14 and 21 pi in all groups. The study demonstrated neither clinical signs, nor gross and histological lesions were observed in all chickens. The isolate induced a significantly (p<0.05) high FAdV antibody titre (2348 ± 1800) at day 21pi in Group B (intraperitoneal) when compared to the Group A (oral) (190 ± 136) in the present of maternal derived antibody (MDA) (7795 ± 1414 at day-old). High antibody response at day 21pi via intraperitoneal route indicates that the viral antigen capable to circumvent MDA from neutralization due to changes in epitopes in L1 loop of hexon and knob of fiber genes. Thus, the live attenuated FAdV isolate (UPM1137CEL35) has high potential for future use against FAdV serotype 8b infection in the chickens.
Keywords | Fowl adenovirus (FAdV), Broiler, Pathogenicity, Immunogenicity, Attenuated.
Received | January 09, 2021; Accepted | January 27, 2021; Published | March 15, 2021
*Correspondence | Norfitriah Mohamed Sohaimi, Faculty of Veterinary Medicine, Universiti Putra Malaysia, Serdang, Selangor, Malaysia; Email: fitriahsohaimi@upm.edu.my
Citation | Sohaimi NM, Bejo MH, Omar AR, Ideris A, Isa NM (2021). Pathogenicity and immunogenicity of attenuated fowl adenovirus from chicken embryo liver cells in commercial broiler chickens. Adv. Anim. Vet. Sci. 9(5): 648-654.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.5.648.654
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Sohaimi et al., This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Fowl adenovirus (FAdV) is non-enveloped DNA virus which has been classified into five molecular species (A-E) and 12 serotypes. Based on International Committee on Taxonomy of Viruses (ICTV) nomenclature, FAdV comprised of groups A (FAdV-1), B (FAdV-5), C (FAdV-4 and -10), D (FAdV-2,-3,-9 and -11) and E (FAdV -6,-7,-8a and -8b) (Benko et al., 2005). FAdV mostly infect broiler chickens at age 3 to 6 weeks old and is readily transmitted both horizontally by the fecal-oral route and vertically by embryonated eggs (Shah et al., 2017; Pan et al., 2017).
FAdV infection caused several clinical diseases in avian species such as inclusion body hepatitis (IBH), gizzard erosion, hydropericardium syndrome (HPS), necrotizing pancreatitis and respiratory disease (Kajan et al., 2013; Okuda et al., 2004; Balamurugan and Kataria, 2004). High pathogenic FAdVs are major viral threat to poultry industry due to high mortality and poor production (Norina et al., 2016; Hair-Bejo, 2005). Among 12 serotypes of FAdV, serotypes 1, 2, 4, 8b,8a and 11 are the most commonly reported as high pathogenic FAdV strains causing outbreaks of IBH, HPS and gizzard erosion in poultry (Schachner et al., 2017; Wang et al., 2019; Juliana et al., 2014; Okuda et al., 2006). To date, FAdV serotype 8b was identified as primary agent of IBH and gizzard erosion outbreaks in Malaysia with significant economic losses in affected chicken farms (Juliana et al., 2014; Norfitriah et al., 2018).
FAdV comprised of major capsid protein encoded for both antigenicity and virulence of virus which is located in hexon and fiber genes (Sohaimi et al., 2019; Zhang et al., 2018). The protruding structure of hexon consist of variable loops which play important role for antibody binding site prior neutralization and has a similar functionality to fiber knob protein (Tomita et al., 2018; Varghese et al., 2004). Nucleotide sequence analysis between FAdV serotypes shown largest variability in the region of L1 loop in hexon and knob in fiber genes (Meulemans et al., 2004). In addition, molecular changes at both regions influence infectivity of FAdV in chickens as a result from viral attenuation in alternative host either chicken embryo or cell cultures (Sohaimi et al., 2019; Sohaimi et al., 2018; Mansoor et al., 2011). In fact, genomic differentiation between pathogenic and non-pathogenic FAdV strains were determined based on fiber gene analysis (Grgić et al., 2014).
Attenuation of FAdV field strains were attempted in chicken embryonated eggs (CEE), hepatoma cells line, Vero cells and recently, in chicken embryo liver (CEL) cells to reduce FAdV virulence following serial passages (Sohaimi et al., 2019; Sohaimi et al., 2018; Ali et al., 2015; Alexandera et al., 1998). Live attenuated FAdV isolates induce long lasting immunity which peak at day 21 post-inoculation (pi) and confer full protection in chickens against IBH by single shot vaccination (Mansoor et al., 2011). A similar observation was reported in previous study in chicken infected by live FAdV isolate via oral route and produced highest antibodies titre at day 21pi (Maiti and Sarkar, 1997). In contrast, the life spans for inactivated vaccine are shortened in the blood circulation and drop rapidly at day 21pi (Mansoor et al., 2011). Furthermore, live attenuated isolate are preferable than inactivated form due to capability of viral antigen to stimulate humoral and cellular mediated immunity in chickens (Schonewille et al., 2010).
In a recent year, IBH outbreaks in poultry producing areas were still reported worldwide and caused by various FAdV serotypes (Jordan et al., 2019). Since IBH is a devastating disease and infects young broiler chicks as early as 7 day of age, thus, control measures are critically important for prevention of virus transmission in a farm. Although strict biosecurity and good management practices might break spreading of viruses between farms, however, high pathogenic strain due to either serotype 4, 8b or 9 could circumvent the system and caused disease outbreak in commercial poultry premises. Thus, it`s urgently need a serotype-specific vaccines against pathogenic FAdV strains as effective control measures since the viral agent is highly resistant and ubiquitous in the farm.
The impact of serial passages of FAdV in cell cultures were the presence of molecular changes in hexon and fiber genes which encoded for virulence determinant (Sohaimi et al., 2019). However, the effect of those changes in broiler chicken remained unknown although FAdV infection commonly caused a disease in meat producing chickens. Therefore, the objective of this study were to determine the pathogenicity and immunogenicity of live attenuated FAdV isolate at 35th passages (UPM1137CEL35) in CEL cells in commercial broiler chickens.
MATERIALS AND METHODS
FAdV isolate
FAdV isolate namely UPM1137 was originated from Malaysian`s commercial layer chicken farms during an outbreak of IBH and gizzard erosion with 2% total mortality. The isolate was characterized molecularly as FAdV group E species with 99% sequence identity and belong to serotype 8b (Sohaimi et al., 2018). Briefly, the isolate used in the present study was propagated in primary CEL cells for 35th passages (UPM1137CEL35) and resulted several molecular changes in hexon and fiber genes (Sohaimi et al., 2019). There were 4 nucleotide (nt) bases substituted within L1 loop region of hexon gene and 3 amino acids (aa) changes at position 44(D-V), 133(S-F) and 185(V-E). In addition, substitution 6nt were detected followed by 2aa changes in knob of fiber genes at position 348(T-P) and 360(A-P). Despite the nt changes, the isolate was remained and classified under serotype 8b and group E species (Sohaimi et al., 2019). The virus titre of the isolate was 106.7 Tissue culture infective dose (TCID)/ml based on Reed and Muench protocol (1938).
Experimental design of pathogenicity and immunogenicity study
Sixty four 1-day-old, Cobb 500 commercial broiler chickens were kept under controlled environment with food and water were given ad-libitum in Animal Research Facility, Universiti Putra Malaysia. The chicks were divided into three major groups namely the groups A, B and C. There are 20 chicks were assigned in both groups A and B, and 24 chicks in the group C. Subsequently, each group was divided further into the sacrifice and mortality groups. For groups A and B, 16 chicks were caged in the sacrifice group and another 4 chicks in the mortality groups. In the group C, 20 chicks were assigned in the sacrifice group and 4 chicks in the mortality groups. At day old (day 0pi), four chicks in the control groups were sacrificed by cervical dislocation, whilst 16 chicks each from groups A, B and C were sacrificed at various interval of time (3, 7, 14 and 21 days pi) of 4 chicks at each sampling time. The chicks in groups A and B were inoculated with 0.5mL live attenuated FAdV isolate (UPM1137CEL35) with virus titre of 106.7TCID/ml via oral route and 106.7TCID/ml via intraperitoneal route, respectively at day-old. The chicks in group C remained uninoculated and acted as the control group. Samples of blood and body weight were collected prior to sacrifice. On necropsy, tissue samples of liver and gizzard were harvested for histopathological examination. The animal study was conducted under approval of Institutional Animal Care and Use Committee (IACUC), Universiti Putra Malaysia with AUP No. R076/2015.
Observation of clinical signs and mortality
All chickens were monitored daily for mortality and clinical signs associated with FAdV infection such as diarrhoea, ruffled feather, depression, weakness and recumbence following inoculation until 21 days pi.
Gross and histopathological examination
On necropsy, the gross lesions were recorded and sample of liver and gizzard were collected and fixed in 10% buffered formalin for histopathological examination. The tissues were then processed, embedded in paraffin wax by 24 hours, and cut into 5µm sections. The sections were stained with Haematoxylin and Eosin (HE) and examined under light microscope (Bancroft and Stevens, 1996).
Detection of FAdV antibody titre
The FAdV antibody titre was determined by commercial Enzyme Linked Immunoabsorbent Assay (ELISA) kit (BioChek, UK). The test was conducted according to manufacture`s protocol. Briefly, serum sample was diluted into 1:100 in sample diluents reagent provided in the kit. The microtitre plate coated with Fowl adenovirus group 1 (FADV Gp1) antigen was added with 100μl of negative and positive control into respective wells as described in the protocol. Then, 100μl of diluted sample was added into the appropriate wells and the plate was covered and incubated at room temperature for 30 minutes. The contents of wells was aspirated and washed with wash buffer (350μl) for 4 times to remove non-specific antibodies and other serum proteins. Anti-chicken IgG labelled with alkaline phosphatase was then added into appropriate wells and incubated at room temperature for 30 minutes. The well was washed with wash buffer (350μl) to remove unreacted conjugate prior added with substrate reagent in the form of p-Nitrophenyl Phosphate (pNPP) chromogen. The plate was incubated for 15 minutes at room temperature. To stop the reaction, 100μl of stop solution was added into appropriate well. The absorbance for control and samples was measured by microtitre plate reader at 405nm wavelength (BioTek EL800, USA). The S/P ratio and antibody titre were analyzed by BioChek software programme.
Statistical Analysis
The body weight and FAdV antibody titre of the chickens were analysed by 1-way analysis of variance (ANOVA) using Statistical Package for the Social Sciences (SPSS) version 22. The significant value was determined at value p<0.05 and subsequently by Tukey honest significance different (HSD) test for significant data (Lee and Lee, 2018).
RESULTS
Clinical signs and mortality
All chickens in the control and FAdV inoculated groups were active and alert without any mortality and clinical sign associated with FAdV infection throughout the trial.
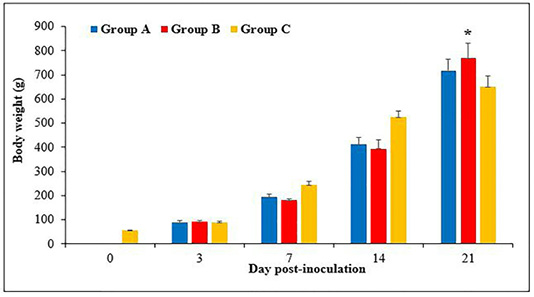
Figure 1: Body weight of chickens following inoculation with live attenuated Fowl adenovirus (FAdV) isolate (UPM1137CEL35) from days 0 to 21 days post-inoculation (pi) in groups A (oral route), B (intraperitoneal route) and C (control) throughout the trial. There are significantly lower (p<0.05) in body weight of chickens in groups A and B at days 7 and 14pi compared to group C. At day 21pi, the body weight of chickens in group B are significantly higher (p<0.05)* than group C. Bar indicated as standard deviation.
Body weight
The body weight of chicks was 55 ± 1.8g at day old and continued to increase to 89 ± 3, 244 ± 8.8, 525 ± 14 and 650 ± 26.8g, at day 3, 7, 14 and 21pi, respectively. The body weight in groups A and B were 90 ± 4 and 93 ± 3g, respectively at day 3pi without significant difference (p>0.05) between groups. At days 7pi, the body weight was 194 ± 6 and 181 ± 3g in groups A and B, respectively, which significantly lower (p<0.05) than group C (244 ± 8.8g). The body weight of chickens at day 14pi was 412 ± 13.7 and 394 ± 18g in groups A and B, respectively, which significantly lower (p<0.05) than group C (525 ± 14g). At day 21pi, the body weight of chickens in groups A and B were 717 ± 24 and 769 ± 31.5g, respectively, which significantly higher (p<0.05) in group B compared to group C (650 ± 26.8g) (Figure 1).
Gross and histological lesions
The liver and gizzard of chickens in all groups were normal at days 0, 3, 7, 14 and 21pi. Liver of chickens were normal in all groups with normal size, brown colour with firm texture, smooth and glistening surface throughout the trial. Histologically, normal architecture of hepatocytes lining by sinusoid without histological changes was observed in all groups (Figure 2 and 3). In gizzard, the koilin layer remained intact in all groups without gross changes from day 0 to 21pi. (Figure 4). Histopathological examination of gizzards revealed intact koilin and epithelium layers without abnormalities in all groups.
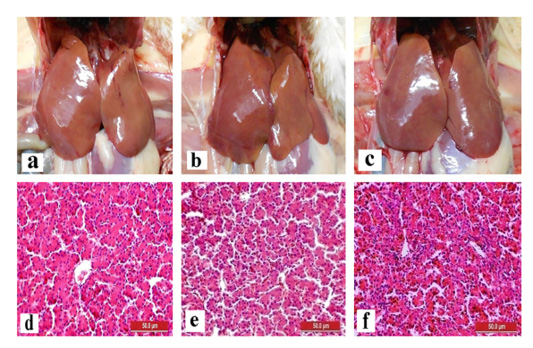
Figure 2: Normal gross and histological findings of liver in all groups of chickens with smooth, glistening surface and normal architecture of hepatocytes lining by sinusoid, respectively, following inoculation with live attenuated UPM1137CEL35 Fowl adenovirus isolate at day 14 post-inoculation (pi). Gross findings indicated from (a) to (c) and histological findings from (d) to (f). (a): Group A inoculated via oral route, (b): Group B inoculated via intraperitoneal (IP) route, (c): Group C: Control, (d): Group A inoculated via oral route, (e): Group B inoculated via IP route, (f): Group C: Control. HE, 40X. Bar = 50µm.
FAdV Antibody response in commercial broiler chickens
The antibody titres were 7795 ± 1414, 4776 ± 1170, 8536 ± 1542 and 2445 ± 1472 at day 0, 3, 7 and 14 days pi, respectively in the control group, but it was not detected at day 21pi (Figure 5). The antibody titres were 4943 ± 2049 and 4188 ± 1310 in groups A and B, respectively, at day 3pi. At day 7pi, the titre were 2243 ± 433 and 3634 ± 2315, in groups A and B, respectively. Subsequently at day 14pi, the
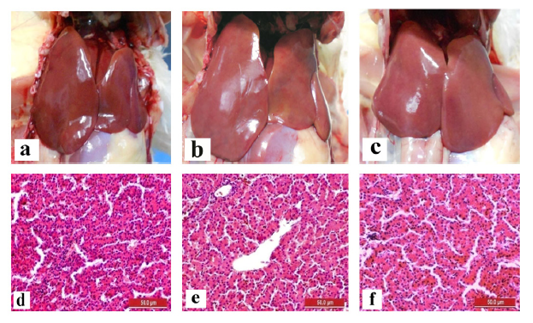
Figure 3: Normal gross and histological findings of liver in all groups of chickens with smooth, glistening surface and normal architecture of hepatocytes lining by sinusoid, respectively, following inoculation with live attenuated UPM1137CEL35 Fowl adenovirus isolate at day 21 post-inoculation (pi). Gross findings indicated from (a) to (c) and histological findings from (d) to (f). (a): Group A inoculated via oral route, (b): Group B inoculated via intraperitoneal (IP) route, (c): Group C: Control, (d): Group A inoculated via oral route, (e): Group B inoculated via IP route, (f): Group C: Control. HE, 40X. Bar = 50µm.

Figure 4: Normal gross finding of gizzard in all groups of chickens with intact koilin layer following inoculation with live attenuated UPM1137CEL35 Fowl adenovirus isolate at day 3 post-inoculation (pi) from (a) to (c) and day 21pi (d) to (f). (a): Group A inoculated via oral route at day 3pi, (b): Group B inoculated via intraperitoneal (IP) route, (c): Group C: Control at day 3pi, (d): Group A inoculated via oral route at day 21pi, (e): Group B inoculated via intraperitoneal (IP) route at day 21pi, (f): Group C: Control at day 21pi.
titre were 2595 ± 1326 and 2866 ± 1281, in groups A and B, respectively. The antibody titre at days 7 and 14pi were not significant difference (p>0.05) between the groups. At day 21pi, the antibody titre of chickens in groups A and B were 190 ± 136 and 2348 ± 1800, respectively. The antibody titre of group B were significantly higher (p<0.05) than groups A and C (control).

Figure 5: Fowl adenovirus (FAdV) antibody response in commercial broiler chickens following inoculation with live attenuated UPM1137CEL35 FAdV isolate in groups A (oral route), B (intraperitoneal route) and C (Control) from day 0 to 21pi. The antibody titre was significantly higher (p<0.05)* in group B than groups A and C at day 21 pi. Bar indicated as standard deviation.
DISCUSSION
The study demonstrated that the FAdV isolate at 35th passage in CEL cells (UPM1137CEL35) is safe and immunogenic in commercial broiler chickens. The isolate could induce antibody with high titre at day 21 pi following intraperitoneal route of inoculation at day-old despite of a high maternal derived antibody (MDA) detected at day-old chicks (7795 ± 1414). Thereafter, the MDA was not detected at day 21pi as a result from normal regression of maternal immunity in chickens (Kowalczyk et al., 1985).
It seems that inoculation of live attenuated FAdV isolates in broiler chickens does not affect overall performance of chickens throughout the trial. The low body weight of chickens at day 7 and 14pi in the oral and intraperitoneal groups could indicates the effect of viral multiplication in target organs (Cook, 1983). Yet, all chickens appeared normal without any abnormal signs in period of the study. A consistent finding reported previously (Junnu et al., 2015).
The study showed that the live attenuated FAdV isolate in CEL cells was safe in broiler chicken. Neither clinical signs, nor gross and histological lesions were recorded in inoculated chickens. It was confirmed that the isolate from passage 35 in CEL cells is attenuated due to presence of molecular changes in hexon and fiber genes which highly associated with virulence determinant as reported in previous work (Sohaimi et al., 2019; Zhang et al., 2018). The current work is consistent with previous finding by utilization of live FAdV-8b vaccine in chickens (Steer et al.,, 2011). As compared to field FAdV isolate, 100% mortality was recorded in infected chickens within 3 to 8 day pi with prominent gross and histological lesions in liver (Ren et al., 2019; Norfitriah et al., 2018; Ahmad et al., 2011; Alvarado et al., 2007). It could be suggested that substitution of amino acids in both genes caused alteration of the proteins as primary attachment site to host cells receptor prior infection. Passaging of field isolate induced molecular changes in hexon and fiber genes, resulting diminished virus virulence as reported in previous paper (Sohaimi et al., 2019).
Current findings are consistent with earlier study following inoculation of mutated L1 loop of hexon gene inoculum at 16th passage adapted embryo without caused any lesion in chickens (Mansoor et al., 2011). In a recent study, the 20th passaged embryo is attenuated in chickens with existence of numerous molecular changes in hexon and fiber genes after continuous passage in CEE (Sohaimi et al., 2018). It seem that substitution of amino acids in L1 loop of hexon and knob of fiber genes in the current FAdV inoculum at 35th passage, influences virus infectivity in broiler chickens and unable to cause disease due to low virus virulence.
High FAdV antibody titre in chicks at day old is due to presence MDA, probably as a result from natural exposure of breeder flocks to FAdV infection since those chickens were not vaccinated against FAdV prior this study (Sohaimi et al., 2018; Alvarado et al., 2007). The antibody titre declined significantly at day 21pi and undetected in chicks. Current study demonstrated that the UPM1137CEL35 isolate could induce high antibody titre at day 21pi in chickens following inoculation with FAdV via intraperitoneal route. As compared to oral route, low antibody titre in chickens perhaps due to neutralization of viral antigen by mucosal immunity lining the gastrointestinal tract (GIT) particularly at gut associated lymphoid tissue (GALT) and failed to replicate effectively in target site for induction of antibody (Muir et al., 2000).
It could be suggested that high antibody response via intraperitoneal route is due to the capability of the viral antigen neutralize MDA and subsequently replicate in target organ to stimulate humoral immune response. The other possible reason is the antigen absorb directly to the blood circulation via intraperitoneal route and traveling to target organ for multiplication without exposure to mucosal immunity in GIT tract (Muir et al., 1995). Additionally, the viral antigen may absorbed into the blood circulation directly, perhaps, able to circumvent MDA from neutralization due to changes in epitopes in L1 loop of hexon and knob of fiber protein in attenuated isolate (Doud et al., 2017). The remaining virus antigen were still exists in the target site, multiplied and continuous replicated to stimulate humoral response. Thus, it leads to induction of antibody response and cause high antibody titre at day 21pi. An active antibody response is not elicited until the MDA titre has dropped below a certain level as reported previously (Saifuddin and Wilks, 1990).
With high antibody response induced by the isolate, it indicates the isolate able to replicate in target organ without cause a disease in chickens. It shown that attenuated isolate strongly induced high antibody response without interference by MDA through parenteral route. Since MDA definitely drops from 14 day of age as reported in various studies, it is the best to provide early protection in young chicks by vaccination against both vertical and horizontal transmission through hatchery vaccination. Implementation of vaccine by systemic route will remain antigen longer in circulation and induced long lasting immunity than oral route as demonstrated in this study. Furthermore, vaccination by injectable route at day old chicks in hatchery is essential to ensure that all chicks will receive adequate amount of vaccine and improve the vaccine efficiency. Previous report demonstrated that mortality was low in vaccinated chickens compared to unvaccinated flocks and thus, vaccination is one of the most effective way to prevent and control disease outbreak in the high risk chicken farms (Afzal and Ahmad, 1990).
CONCLUSION
The UPM1137CEL35 FAdV isolate is safe and could induce high antibody titre in broiler chickens when inoculated via intraperitoneal route. Thus, the isolate has high potential for future use in the prevention and control of FAdV serotype 8b infection in chicken farms.
ACKNOWLEDGEMENTS
The study was funded by Ministry of Education, Malaysia, and Ministry of Science and Technology, Malaysia with grant numbers 6369101 and 6364002, respectively. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of INTEREST
The authors declares that they have no competing interests.
authors contribution
Conceptualization: Mohd Hair Bejo, Data curation: Norfitriah Mohamed Sohaimi. Formal analysis: Norfitriah Mohamed Sohaimi, Methodology: Norfitriah Mohamed Sohaimi, Mohd Hair Bejo, Software: Norfitriah Mohamed Sohaimi,Validation: Mohd Hair Bejo, Abdul Rahman Omar, Aini Ideris, Nurulfiza Mat Isa, Investigation: Norfitriah Mohamed Sohaimi, Writing - original draft: Norfitriah Mohamed Sohaimi Writing - review & editing: Norfitriah Mohamed Sohaimi, Mohd Hair Bejo, Abdul Rahman Omar, Aini Ideris, Nurulfiza Mat Isa
REFERENCES





