Advances in Animal and Veterinary Sciences
Research Article
Early Effects of Experimental Infection of Sheep with Erwinia persicina
Walaa I. Mohamaden1,2,3, Zhang Zhen-Fen2,3, Ibrahim M. Hegab4, Shi Shang-Li2, 3*
1College of Grassland science, Gansu Agricultural University, Lanzhou 730070, China; 2Key Laboratory of Grassland Ecosystem of the Chinese Ministry of Education, Lanzhou 730070, China; 3Department of Animal Medicine, Faculty of Veterinary Medicine, Suez Canal University, Ismailia 41522, Egypt;4Department of Hygiene, Zoonosis and Animal Behavior & Management Faculty of Veterinary Medicine, Suez Canal University, Ismailia 41522, Egypt.
Abstract | Erwinia persicina is a gram-negative pathogenic bacterium that can infect plants and has been isolated from human urine. This study aimed to investigate the effect of E. persicina on small ruminants. In the current study, twenty growing sheep were divided into two groups: a control group (n=10) and an E. persicina-injected (Ep) group (n=10). The Ep group was subcutaneously injected with a single dose of E. persicina bacterial suspension. The animals were carefully examined, and blood and serum samples were collected from both groups at 0, 2, 4, 8, 24 and 48 hpi time points. Clinical examination showed signs of apathy, loss of appetite and ruminal stasis in the Ep group. There were significant decreases in the proinflammatory cytokines (IL-6, IL-1β and TNF-α) and the anti-inflammatory cytokine IL-10 in the Ep group compared to the control group. White blood cell counts were significantly increased at 24 hpi in the Ep group. The immunohistochemical investigation of Toll-like receptor 4 (TLR4) in the lymph nodes did not show any significant differences between the Ep and control groups. E. persicina might have an immunomodulatory effect on sheep, but the mode of infection and the virulence of these bacteria require further investigation.
Keywords | Erwinia persicina, ELISA, Histopathology, IHC, Sheep
Received | September 14, 2020; Accepted | October 24, 2020; Published | March 01, 2021
*Correspondence | Shi Shang-Li. Department of Animal Medicine, Faculty of Veterinary Medicine, Suez Canal University, Ismailia 41522, Egypt; Email: shishl@gsau.edu.cn
Citation | Mohamaden WI, Zhen-Fen Z, Hegab IM, Shang-LI S (2021). Early effects of experimental infection of sheep with erwinia persicina. Adv. Anim. Vet. Sci. 9(5): 623-630.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.5.623.630
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Shi et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
In nature, plants and animals may functionally share viral, fungal or bacterial infections, but the majority of the bacterial pathogens are usually specific to a limited number of host organisms. Nevertheless, gram-negative pathogenic bacteria are prokaryotic families infamous for using various strategies to invade and colonize both plant and animal hosts. For example, some bacterial strains, such as Pseudomonas aeruginosa PA14, have the ability and virulence to infect a diverse range of hosts that include both plant and animal species (Büttner and Bonas, 2003).
Erwinia is classified as a genus of the Enterobacteriaceae. Erwinia cells are gram-negative rods that are propelled by peritrichous flagella and can ferment glucose aerobically to produce acid (Kado, 2006). Erwinia species can cause necrosis, tissue maceration, vascular wilt and tissue hypertrophy to different parts of different species of plants (Kado, 2006). E. persicina can survive in water and soil (Zhang and Nan, 2014), and it can produce considerable quantities of slime composed of proteins, exopolysaccharides (EPSs) and water (Kiessling et al., 2005). These components give this species some antigenic characters, but these characters are not well understood. In plants, E. persicina causes necrosis in red-brown fruits (Hao et al., 1990) and wilting in lucerne (Zhang and Nan, 2014). Erwinia species has been reported to be a human pathogen in three case reports; E. persicina was isolated from the urine of urinary tract infected woman (O’Hara et al., 1998), the same strain induced pathological lesions in mice after experimental infection (Mohamaden et al., 2019), other species of Erwinia were reported in pathological in humane (Prod’homme et al., 2017). However, the reported cases were cured and treated with a simple course of antibiotics. Despite the recorded pathogenic effects of some Erwinia species on human health, scientists have discovered a new treatment for acute lymphocytic leukemia from an E. carotovora subspecies; Erwinia chrysanthemi. It can produce asparaginase, which is used to suppress the immune system and the mitotic division of lymphocytic tumor cells (Papageorgiou et al., 2008).
The immune system is a system of biological structures and processes within an organism. It plays a major role in the prevention of disease (Nicholson, 2016). Multiple research studies have focused on the activities of some bacteria producing EPSs and their effects on living animal tissue. They discovered that bacterial EPSs have antitumor (Colliec Jouault et al., 2001) and immuno-stimulatory activities (Arena et al., 2006). Additionally, EPSs from lactic acid bacteria exhibit macrophage activation abilities and mitogenic activity (Surayot et al., 2014), as well as the ability to enhance or inhibit the production of proinflammatory cytokines (Shin et al., 2016; Murofushi et al., 2015), revealing their fluctuating immunomodulatory actions. However, there are studies that have used sheep as an experimental animal for the detection of the immune responses triggered by different methods, such as by receptors and proinflammatory cytokines (Kowalewska et al., 2017; Yao et al., 2017; Shui et al., 2018). Some researchers have reported findings from sheep exposed to slime-producing bacteria, including those with exopolysaccharide membranes (Pérez et al., 2009).
In this work, we investigated the ability of the plant pathogen E. persicina to modulate the immune response of sheep by detecting the levels of TLR4 and some pro-inflammatory and anti-inflammatory cytokines. In addition, we evaluated the health condition of the animals during the early hours of infection.
MATERIAL AND METHODS
Animals and Experimental Design
This study was approved by the institutional animal care and use committee, College of Grassland Science, Gansu Agriculture (GSC-IACUC-2018-0014). Twenty short-tailed Han sheep aged approximately 6-9 months were purchased from a licensed supplier. Food was provided at specific time and water ad-libitum.
Animals were randomly allocated into two groups: the Ep group (n=10, five males and five females) (injected with 1.3×109 CFU/ml of Erwinia persicina (strain Cp2, GenBank accession JN900058) bacterial suspension based on our previous work (Mohamaden et al., 2019) and the saline group (n=10, six males and four females) (injected with 0.9% NaCl saline). All animals were injected subcutaneously with a total of 10 ml divided into 5 ml doses on each side of lateral abdominal wall. Animals were initially examined prior to the start of the experiment for any signs of illness and monitored for any clinical findings or behavioral signs throughout the experiment.
Five milliliters of blood samples were collected from the jugular vein of each animal at specific time points 0 h, 2 h, 4 h, 8 h, 24 h and 48 h post-injection (hpi) through a venous catheter. One milliliter of blood was withdrawn and directly placed into a sodium-EDTA-containing tube for hematological examination, and the rest was poured into a serum collection tube for serum separation for further analysis.
Three animals from each group were anesthetized for the surgical collection of the prefemoral lymph nodes at 4 hpi. The lymphatic tissues were collected then washed with saline to remover blood, then the lymph node was cut into smaller pieces and fixed in 10% PBS formalin for histological and immunohistochemical studies. All animals were monitored for four weeks after the end of the experiment including physical examination, complete blood count and medical treatment and care for those were used in the surgery.
Bacteriological Examination
Buccal, nasal, fecal, vaginal, and preputial bacterial swabs were collected at 24 and 48 hpi from all the animals and cultured on maltose nutrient agar (Zhang and Nan, 2014) for detection of E. persicina.
Hematological Examination
A Sysmex pocH-100iV Diff ™ Automated Hematology Analyzer (Japan, catalog Number: Chuo-Kobe 651-0073) was used for analysis of the complete blood values including hemoglobin level, RBC count and differential leukocytic count.
Serum Levels Of Il-6, Il-10, Il-1Β And Tnf-Α
The serum interleukin-6 (IL-6), interleukin-10 (IL-10), interleukin-1beta (IL-1β) and tumor necrosis factor-alpha (TNF-α) levels at each time point were measured using an enzyme-linked immunosorbent assay (ELISA) using goat-specific kits following the manufacturer’s instructions (ShangHai Lengton Bioscience Co. China).
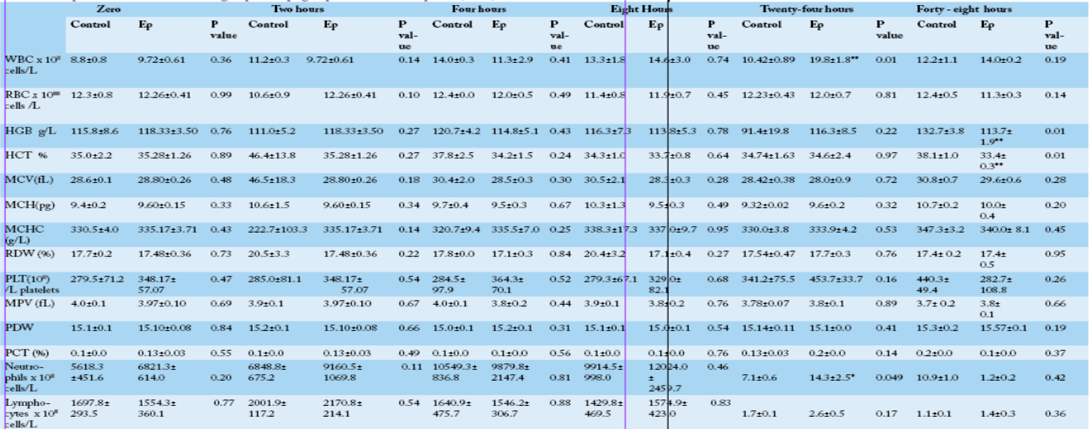
Hemoglobin (HGB), Hematocrit (HCT), Mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH) and mean corpuscular hemoglobin concentration (MCHC), Red blood cell distribution width (RDW), platelet counts (PLT), mean platelet volume (MPV), platelet percent (PCT ) and platelet distribution width (PDW).
* Significant at p ≤ 0.05, ** Significant at p ≤ 0.01

Figure 1: White blood cells count at 0 h, 2 h, 4 h, 8 h, 24 h and 48 h post-injection in the control and Ep groups. The average values (mean ± S.D.) are shown. Asterisks (**) indicate significant differences with a significance level of p ≤ 0.01.
Histopathological Examination
Fixed tissue samples were processed for dehydration in increasing concentrations of ethanol, followed by two changes of xylene, and then embedded in paraffin wax. Subsequently, the paraffin blocks were sectioned serially at 4 mm using a Leica RM 2235 microtome (Leica Bio-systems, Germany). All serial sections were stained with routine hematoxylin and eosin, washed with tap water, dehydrated, and mounted with neutral gum.
Expression of Toll-Like Receptor 4 (Tlr4)
Tissue sections were processed for immunohistochemical detection and quantification of toll-like receptor 4 (TLR4) expressions. Immunoreactivity was detected using a 1:100 dilution of purified mouse monoclonal anti-TLR4 antibody (ab22048 Abcam, USA). All stained sections were examined under a light microscope (Axio Imager A2, ZEISS). Images were captured using an Axiocam MRC5. The integrated optical density (IOD) was then measured using Image pro-plus software.
Statistical Analysis
All statistical analyses were performed using SPSS 22.0 software (Armonk, NY: IBM Corp). Each time point was compared using a paired two-tailed Student’s t-test between the control and EP groups. A two-way analysis of variance (ANOVA) with group and time as fixed variables was also performed to explore both main and group × time interactions effects on the different hematological parameters and pro-inflammatory cytokine levels as dependent variables. If significant main or interaction effects were found at p ≤ 0.05, Duncan’s post hoc test was applied.
RESULTS
Clinical Finding and the Bacteriological Culture
Normal body temperature was recorded in both the Ep and
control groups. The clinical signs at different time points of the treatment are shown in Figure (1) and Table (2). The bacteriological culture results did not show any growth of E. persicina.
Hematological Findings
Comparison between the control and Ep groups at different time points showed that there was a significant increase in the total leukocyte count in the Ep group at 24 hpi concomitant with a significant increase (p=0.049) in
Supplementary Table (1): Complete blood count of control group and Ep- group at different time points (Two-Way ANOVA)
| Hematological variables | Group Effect | Time effect | Group × Time interaction | |||
| F | P | F | P | F | P | |
|
WBC x 109 /L cells |
1.51 | 0.23 | 1.04 | 0.41 | 0.78 | 0.57 |
|
RBC x 1012 /L cells |
0.04 | 0.83 | 0.64 | 0.67 | 1.15 | 0.35 |
| HGB g/L | 0.05 | 0.83 | 1.08 | 0.39 | 1.33 | 0.27 |
| HCT % | 2.99 | 0.09 | 1.14 | 0.36 | 0.92 | 0.48 |
| MCV (fL) | 3.24 | 0.08 | 1.93 | 0.11 | 1.79 | 0.14 |
| MCH (pg) | 2.03 | 0.16 | 1.27 | 0.30 | 0.86 | 0.52 |
| MCHC (g/L) | 2.89 | 0.10 | 2.36 | 0.06 | 2.25 | 0.07 |
| RDW (%) | 3.50 | 0.07 | 1.17 | 0.34 | 1.39 | 0.25 |
|
PLT 109 /L platelets |
0.77 | 0.39 | 0.64 | 0.67 | 0.80 | 0.56 |
| MPV (fL) | 0.12 | 0.73 | 1.29 | 0.29 | 0.26 | 0.93 |
| PDW | 0.28 | 0.60 | 3.21 | 0.02 | 1.24 | 0.31 |
| PCT (%) | 0.84 | 0.36 | 0.37 | 0.87 | 0.37 |
0.87 |
Table 2: Clinical findings at different time points and bacteriological culture results at 24hpi and 48hpi of control group and Ep group.
| Ep. Group. | Control group | Time points |
| Normal temperature, normal appetite, alert, active, normal respiration, and heart rate. |
Normal temperature, normal appetite, alert, active, normal respiration, and heart rate. |
0-hpi |
| apparent dullness in response to external stimuli, loss of appetite, apathy, social isolation in the corner of the sheep pen and normal gait when forced to move. |
2-hpi
|
|
|
anorexia, dullness, depression shallow rapid respiration with tachycardia, |
4-hpi | |
| Anorexia, dullness and depression, mild distention of rumen, ruminal stasis | 6-hpi | |
| Anorexia, rapid respiration and tachycardia, ruminal stasis, free- gas bloat. Introduction of the stomach tube into the rumen to liberate free gases was necessarily applied for one animal, while the rest of the animals recovered without intervention. | 8-hpi | |
|
Normal temperature, all the clinical findings subsided, the rumen motility was restored, and the animal returned to eating normally. |
24-hpi | |
|
Normal temperature, normal appetite, alert, active, normal respiration, and heart rate. Negative E. persicina culture. |
48-hpi |
the neutrophil count in the Ep group (14.307±2.511) compared with the control group (7.050± 0.643) (Table 1). The hematocrit and hemoglobin values also showed significant decreases (p≤0.05) in the Ep group at 48 hpi, while other hematological parameters did not show any significant differences between groups at different time points (Table 1). Statistically, neither group, time nor group × time interaction showed any significant influence on any of the examined blood parameters except for the platelet distribution width (PDW), which was influenced by time (F5,50= 3.21, P=0.02) (Supplementary Table 1), where the highest value was recorded at 48 hpi.
Serum Il-6, Il-10, Il-1Β and Tnf-Α Levels
The group × time interaction did not show any significant effect on the serum levels of IL-10 (F5,67= 1.52, p=0.20), IL-6 (F5,67= 2.31, p=0.55), IL-1β (F5,67= 1.61, p=0.17) and TNF-α (F5,67= 1.11, p=0.37). t-test analysis between the two groups revealed non-significant differences in the IL-10 serum levels between the control and Ep groups, but
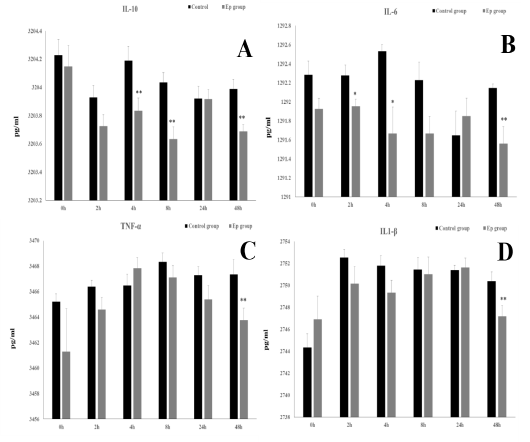
Figure 2: Mean serum cytokine levels [IL-10 (A), IL-6 (B), TNF-α (C) and IL1-β (D)] at 0 h, 2 h, 4 h, 8 h, 24 h and 48 h post-injection in the control and Ep groups. Asterisks (* and **) indicate significant differences with significance levels of p ≤ 0.05 and p ≤ 0.01, respectively.
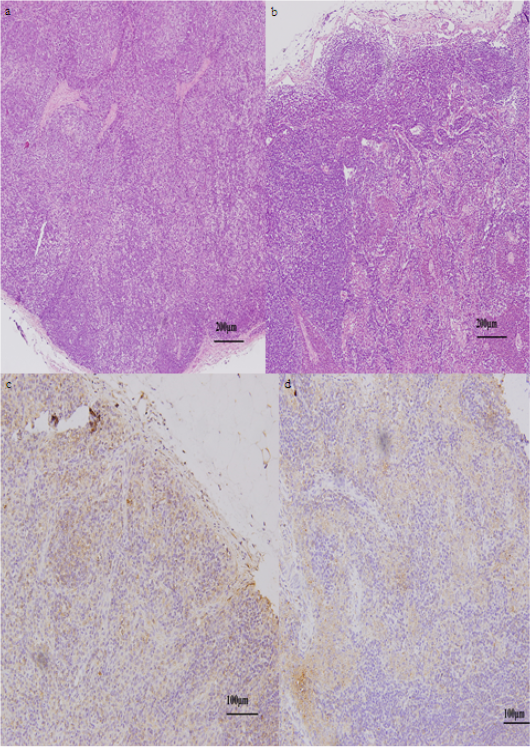
Figure 3: Lymph nodes of sheep at 4 hpi. (a). Normal histoarchitecture of lymphatic tissue of the control group. (b) Liquefactive degeneration found in some areas of the lymph node of the Ep group. Immunohistochemical expression in the control (c) and Ep (d) groups, (brown) as TLR4 stanning and (blue) as cells nuclei.
at 4 hpi (t10=2.61, p=0.03), 8 hpi (t10=3.62, p=0.03) and 48 hpi (t10=3.65, p=0.004), the IL-10 serum levels were significantly lower in the Ep group than in the control group (Fig. 2A). IL-6 showed a significant decrease in the serum of Ep sheep at the early 2 hpi (t10=2.45, p=0.035) and 4 hpi (t10=3.01, p=0.013) time points and later at 48 hpi (t10=3.23, p=0.009) compared to the control group (Fig. 2).
IL-1β and TNF-α only showed significant decreases (IL-1β: t10=2.45, p=0.034, and TNF-α: t10=2.42, p=0.04) at 48 hpi in the serum levels of the Ep group (Fig. 2).
H&E and Immunohistochemistry
Lymph nodes were collected from both groups 4 h after injection. Results of the histopathological examination showed aggregation of inflammatory cells and lymphocytes in the subcortical sinus beside degenerated cells in the lymph node of the Ep group (Fig. 3 a & b). Statistical analysis of toll-like receptor 4 (TLR4) expressions in the lymphatic tissue at 4 hpi did not show any significant difference between the control and Ep groups (0.16±0.01 and 0.15±0.00, respectively) (Fig. 3 c & d).
DISCUSSION
Several studies focused on sheep infections by gram-negative bacteria by challenging animals with soil-surviving and/or plant pathogens either experimentally (Knappe-Poindecker et al., 2014) or naturally (Leitner and Krifucks, 2007). The present study is dealing with the early effects of invading plant pathogen into the livestock which is different in the structure from most of the gram-negative bacteria been studied. In this study, E. persicina-infected animals showed dullness, loss of appetite, tachycardia, tachypnea, and ruminal stasis. All these signs are common systemic inflammatory response syndromes (SIRS) similar to typical signs of mild infection with bacteria (Peter et al., 2017). The animals recovered from all of these symptoms, and the rumen motility was restored after 24 hpi, which indicates the low virulence of these bacteria Leukocytes form a defensive, movable army that protects the body from damage induced by bacteria, viruses, parasites, and tumor cells. Previous studies have indicated that bacterial biofilms stimulate leukocyte proliferation (Sykes, 2014). The CBC results showed that there was a gradual increase in the white blood cell count during the experiment, although there were no significant differences between groups, except at 24 hpi; these results have been reported previously in animals infected with gram-negative bacteria (Sykes, 2014). The WBC count increase might be attributed to bacterial DNA and/or the biofilm chemical structure, which contains 7.6% proteins. Similar results obtained by Chen and Wen (2011) who recorded a significant increase in the peripheral WBC count at 10 and 24 hours after intravenous infusion of the bacterial endotoxin Lipid into sheep.
The immune system reacts towards bacterial biofilms in a complicated and contradictory manner. Bacterial biofilms can stimulate or inhibit the immune response of the host against bacterial invasion depending on the immune status of the host, the host species, the composition of the biofilm and other factors (Watters et al., 2016). IL-10, which is considered the most important anti-inflammatory cytokine (Asadullah et al., 2003), acts generally as an immunosuppressive molecule, with its main biological function which is the limitation and termination of inflammatory responses and the regulation of differentiation and proliferation of several immune cell types (de Moreno et al., 2011). IL-6, TNF-α and IL-1β are mainly produced by activated macrophages, T lymphocytes, and NK cells, and their function is to stimulate the cellular immune process and enhance the inflammatory response to eliminate abnormal cells (Liu et al., 2009). Our results revealed that E. persicina suppressed the production of interleukin-10 (IL-10) at 4, 8 and 48 hpi, which might suggest an evoked inflammatory condition. Nevertheless, the results also showed an early significant decrease in the IL- 6 levels in the serum of the Ep group at 2 and 4 and a later decrease at 48 hpi, while IL-1β and TNF-α were significantly reduced at 48 hpi. Unlike other studies that used lipopolysaccharides (LPS), which are highly virulent endotoxins to sheep that lead to a significant release of proinflammatory cytokines into the peripheral circulation (Byrne et al., 2018), the inhibited production of proinflammatory cytokines might be due to the bacterial exopolysaccharide produced by E. persicina that can suppress the general immune response. Murofushi et al. (2015) demonstrated that certain exopolysaccharides in Lactobacillus plantarum biofilms decreased the production of the proinflammatory cytokines interleukin-6 (IL-6), interleukin-8 (IL-8), and monocyte chemotactic protein-1 (MCP-1) by increasing the production of negative regulators of Toll-like receptor-4 (TLR4), an important pattern recognition receptor (PRR) for the detection of pathogen-associated molecular patterns (PAMPs). Additionally, it is important to mention that E. asparaginase, another species of the genus Erwinia, produces an asparaginase that significantly decreases the secretion of TNF-α and IL-6 from macrophages even when stimulated by incubation with interferon gamma (IFN-γ) or with LPS (Song et al., 2017). Therefore, E. asparaginase has recently been utilized as a treatment for acute lymphoblastic leukemia (Pieters et al., 2011) due to its immunosuppressive effect.
When all the clinical symptoms started to show at 4 hpi, lymphatic tissue was collected to assess the early effect of E. persicina bacterial injection on the tissue histology and on the expression of TLR4. The histopathological findings showed some areas of cellular degeneration in the lymph nodes, while the immunohistochemical results did not show any significant difference in the expression of TLR4 in lymph nodes between the Ep group and the control group at 4 hpi. This result indicates that E. persicina in the given concentration can induce some pathological lesions even in the early stages but did not provoke significant TLR4 expression.
CONCLUSION
E. persicina is a pathogenic plant bacterium that survives well in the environment around livestock. E. persicina may not be able to live and propagate inside sheep tissues. However, the chemical and microbial products of E. persicina have pronounced effects on sheep health conditions. Infection of sheep with E. persicina induced clinical manifestations that subsided quickly, indicating low virulence. The bacteria suppressed the immune system by inhibiting the production of some inflammatory and anti- inflammatory cytokines. Therefore, it is necessary to investigate these bacteria further to study every microbial product and to investigate its effect on living animal tissue, which may lead to medical and practical discoveries in the future.
ACKNOWLEDGMENTS
This study was supported by the National Nature of Science Foundation of China (31460639), the National Forage Industry Technology System Project (CARS-34) and The Scientific Research Innovation Team for Controlling Grassland Pest Rodents.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
authors contribution
Walaa I. Mohamaden designed the experiment, carried out the experiment. Zhang ZhenFen provided the bacteria and carried out the experiment. Ibrahim M. Hegab helped in lab work and statistical analysis. Shi Shang-li approved the experiment and provided the financial support. All authors assisted in writing the manuscript.
References