Advances in Animal and Veterinary Sciences
Research Article
Cross-Sectional Study on Dermatological Affections of Companion Animals Caused by Dermatophytes and other Keratinophilic Fungi in Greater Cairo Area, Egypt
Hassan A. Hassanien1, Rafik H. Sayed2, Heidy S. Abo Elyazeed1*
1Department of Microbiology, Faculty of Veterinary medicine, Cairo University; 2Institute for evaluation of veterinary biologics (CLEVB), Agricultural Research Center (ARC), Abbasia, Cairo, Egypt.
Abstract | The colonization of fungal organisms on different parts of the integumentary system of a wide range of animal species is very common as a part of normal dermal mycobiota or through infection. Primary fungal colonization is mainly caused by different dermatophytes, while secondary fungal colonization is caused mostly by non-dermatophyte keratinophilic fungi and was found to be associated with particular host immune system-related disorders. The present cross-sectional study has been designed to determine the diversity of fungal species that are associated with different dermatological affections in companion animals, including dogs, cats, parrots, horses, guinea pigs, rabbits, rats, donkeys, cows, and goats. The study was conducted through a year (June/2019 - June/2020) on 600 skin samples (nail, hair, scales, and skin scraps) collected from companion animals all over the Greater Cairo Area (GCA), Egypt. A wide range of fungal species have been recovered, and out of the 600 samples, 85 positive fungal cultures have been identified (14.16%). The isolated fungal species included 30 dermatophyte species (24 Microsporum canis, 2 Microsporum gypseum, 1 Trichophyton equinum, 2 Trichophyton mentagrophytes, and 1 Trichophyton verrucosum), 10 isolates of Malassezia pachydermates, 33 Candida species (11 Candida albicans, 21 Candida tropicalis, and 1 Candida glabrata), 1 Alternaria alternata, 4 Penicillium species, 5 Aspergillus niger, 1 Fusarium chlamydosporum, and 1 Fusarium oxysporum.
Unfortunately, most of the fungal species that have been recovered from the companion animals in the present study zoonotic and constitute a human public health hazard.
Keywords | Dermatomycoses, Dermatophytes, Companion animals, Cutaneous zoonosis, Cutaneous mycoses.
Received | January 21, 2021; Accepted | January 26, 2021; Published | February 20, 2021
*Correspondence | Heidy S Abo Elyazeed, Department of Microbiology, Faculty of Veterinary medicine, Cairo University, Egypt; Email: heidyshawky86@hotmail.com
Citation | Hassanien HA, Sayed RH, Elyazeed HSA (2021). Cross-sectional study on dermatological affections of companion animals caused by dermatophytes and other keratinophilic fungi in greater cairo area, egypt. Adv. Anim. Vet. Sci. 9(4): 615-622.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.4.615.622
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Elyazeed et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Companion animals and its human association started 10.000 years ago with dogs and cats. By the time, a wide range of animal species have been incorporated in this human/animal companionship, and their relationships with human societies have been significantly increased. Nowadays, a pet animal can be considered a family member. However, companion animals can be a source of a wide range of viral, bacterial, parasitic, and fungal zoonotic emerging and re-emerging diseases (Chomel, 2014).
Dermatomycosis is one of those neglected zoonotic diseases transmitted from animals to humans. It is caused by various fungal species that extend from the zoophilic fungi M.canis to different yeast causes, including C.albicans, non-albicans candidae, and Malassezia pachydermatis. The latter species is the only lipophilic Malassezia spp. Causing severe inflammatory cutaneous lesions in man than the anthropophilic fungal agents (Nenoff et al., 2012).
Humans can harbor dermatophyte infections from a wide range of companion animals. Infected dogs and cats are major transmitters, especially in the case of cats as an asymptomatic carrier of M. canis. Also, equines with persistent outbreaks of T. equinum due to the relative unhygienic condition of fomites associating grooming within stables can act as an essential source of human infection. Nevertheless, T. verrucosum infected cows transmit the disease (Bond, 2010). Even rodents (rats, rabbits, hamsters, and G. pigs) have been reported to be the main source of the zoophilic T.benhamiae transmission to humans (Tekin et al., 2014).
Although dermatophytosis in companion animals is the most common dermatomycotic infection, other non- dermatophytes cutaneous fungal affections are characterized by alopecia, scaling, and crusting that mimics the dermatophyte lesions have been reported. These infections are caused by other mold or yeast fungi (Allizond et al., 2016). These non-dermatophytes fungal agents with prominent dissemination through animal populations are increasingly reported as potentially infectious agents. It shows several pathogenic capabilities, variable keratinophilic activities and can lead to several severe diseases in both man and animal (Seyedmousyi et al., 2015). By searching the PubMed research database, it was found that up to 92 studies were performed on dermatomycoses in pets through the period between 1962 and 2019. Of those studies, 82.6% have been performed in the last ten years Figure (1). This is clear proof of the increased importance of studies on different fungal causes of dermatomycosis in companion animals in different parts of the world.
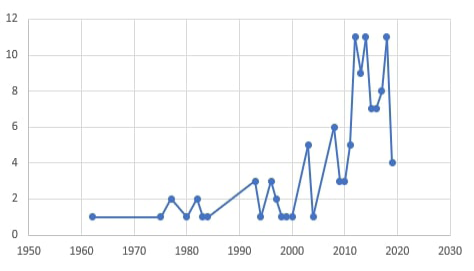
Figure 1: The count of research studies directed toward “Dermatomycoses in pets” through the period between 1962 and 2019.
The present study aimed to conduct cross-sectional studying and evaluation for the epidemiological distribution and occurrences frequencies of different fungal causes associated with various dermatomycotic infections in companion animal, which positively impact public health awareness and constitute a preliminary step for further epidemiology related investigations or treatment, vaccine, and new diagnostic techniques related studies.
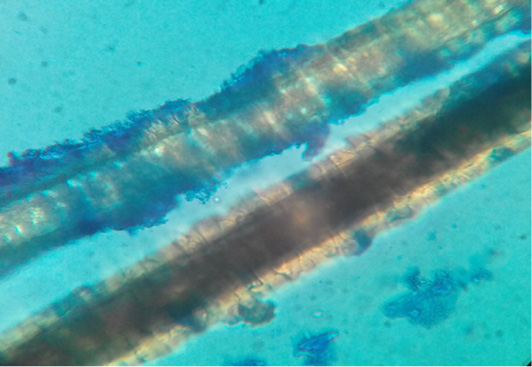
Figure 2: Dog hair sample stained with Lactophenol cotton blue examined microscopically (40X) showing ectothrix hair invasion with small spores.
MATERIAL AND METHOD
Study design
The current cross-sectional study was designed to describe the state of cutaneous mycotic infection in companion animals in the Greater Cairo Area-Egypt. The study’s purpose was determining the prevalence of dermatomycoses and configuring the various correlation of the rate of infection and other risk factors. The population that was chosen for the study was the different companion animal species suffering from cutaneous affections in Greater Cairo Area Egypt. The study was held as follows; Sample size calculation, study population determination, sample collection, isolation and identification of different fungal causes, and statistical analysis.
Sample size calculation and study population
Sample size was estimated based on the following formula; n= (Z1- α/2)2.p (1-p)/ d2 where (n) was the number of samples, (Z1- α/2) was the standard normal, (p) was the expected proportion in the population, (d) was the absolute precision (Charan and Biswas, 2013). A 5% precision was used, 1.96 standard normal, and 48% expected proportion in population relied on a previous study (Dworecka-Kaszak et al., 2020). At least 457 samples were required to achieve appropriate precision.
The skin samples were collected by expertise veterinarians from companion animals in veterinary clinics, animals ‘shelters, and animals’ farms from all over the Greater Cairo Area (GCA), Egypt. A total of 600 samples (310 dogs, 237 cats, 4 horses, 3 parrots, 2 Guinea pigs, 25 rats, 12 rabbits, 1 donkey, 5 cows, and 1 goat) were collected between June 2019 and June 2020 and examined. All samples were collected from animals with skin lesions, including alopecia, circumscribed hair loss, and itchy scaly cutaneous areas. Most of the sampled animals were not treated with an antifungal drug before sampling.
Samples collection
All the skin samples were collected using the following standardized technique: the lesions were firstly cleaned by 70% ethyl alcohol. Using a sterile scalpel or curette, the skin was scraped from the active peripheral border of the lesion or any damaged looked hair. Also, stumps of hair are pulled out using sterile epilator forceps as a second most useful specimen. The collected samples were collected in sterile plastic Petri dishes and cups (Quinn, 1994).
Isolation and identification of different fungal causes:
Direct microscopic examination: Hair, chopped nails, or skin scraping were placed on a sterile slide. Few drops of potassium hydroxide (KOH) with dimethyl sulfoxide (DMSO, Serva®) and glycerin (Serva®) were added to the sample (DMSO enhance KOH 10-20% clarifying ability). The slide was covered with a coverslip, heated gently, left in humid chamber 20-30 minutes, and examined using low and high dry power lenses of a bright-field light microscope (Olympus CKX41 microscope with camera model C-7070).
Primary mycological isolation: Irrespective of the direct microscopic results (positive or negative), the samples were cultured at least three times (Enany et al., 2013) by placing the hair, chopped nails, scales, and skin scrapes on the surface of prepared slant agar under complete aseptic condition. The following media were used for isolation:
Sabouraud’s dextrose agar (SDA, Oxoid®) supplemented with Chloramphenicol (Oxoid®) 0.5 g/L as a broad-spectrum antibiotic and Cycloheximide (Oxoid®) 0.5 g/L as an anti-saprophytic fungal agent: for isolation of Epidermphyton Spp and Microsporum Spp, at 25⁰C for 21 ds up to 30 ds, Sabouraud’s dextrose agar (SDA, Oxoid®) with Chloramphenicol 0.5 g/L and Cycloheximide (Oxoid®) 0.5 g/L and enriched with thiamine and inositol (Serva®) for isolation of Trichophyton verrucosum, at 37⁰C for 30 ds, Sabouraud’s dextrose agar (SDA, Oxoid®) with Chloramphenicol and Cycloheximide enriched with nicotinic acid (Serva®) for isolation of Trichophyton equinum, at 25⁰C for 21 ds up to 30 ds, Sabouraud’s dextrose agar (SDA, Oxoid®) with chloramphenicol only for isolation of different non-dermatophytes fungal species.
The broad-spectrum antibiotic was added to the isolation medium to prevent the growth of contaminating bacteria and enhance the chances of pure primary isolation of the fungal elements, as well as the anti - saprophytic fungi, were used to prevent the growth of the rapidly growing saprophytic fungi, giving better chances to the different dermatophytes pure primary isolation.
All inoculated slants were examined weekly for the fungal growth, and those showing any growth were sub-cultured into new slant agar for refrigeration preservation and, at the same time, were further identified, while those without growth were left to complete the whole incubation period (Quinn, 1994).
Further identification:
At this stage, the resulting culture outcome was further examined using different techniques that depend upon the primary identification of fungal isolates. The chromogenic agar (Himedia®) was used to identify the different Candida species (Ghelardi et al., 2007), while the microculture technique was adopted to identify the different dermatophytes species and to confirm the previously identified non-dermatophyte mold species.
The microculture technique was done according to (Nugent et al., 2019); 4 edges of the agar block were inoculated with the fungal isolate to be identified, and 1ml of sterile water is added to the bottom of the plate to keep it in a humid condition. The agar blocks were incubated at 37⁰C in the case of T. verrucosum and 25⁰C in the case of other suspected dermatophytes. The inoculated agar blocks were regularly examined, and sterile water was added to avoid dryness of the microculture. Once the inoculated edges of the microculture blocks start showing growth, the slide is examined microscopically for the presence of characteristic microscopic fungal elements, e.g., macroconidia, microconidia, spirals, chlamydospores. The coverslip was transferred to a new sterile slide, and the new sterile coverslip was placed on the exposed microculture block. The culture on the coverslips was stained with Lacto-phenol cotton blue (LPCB, Himedia®) and examined by a 40x objective lens of bright-field light microscope (Olympus CKX41 microscope with camera model C-7070).
Statistical analysis
All data visualization parameters and charts, statistical analysis tests, and plots have been performed using different R program codes previously existed and created for data entry, variables identification, analysis, and blotting (R program, 2013) in Figure (4), as well as the drop down menus of the Excel analytical functions in Figures (1 and 3).
Results
The results revealed that of the 600 examined samples, 85 positive cultures (14.16%) had been identified. The isolation frequencies of each fungal species from the total ex
Table 1: Frequencies of occurrence of fungal species indifferent animal species.
| Different Companion animal species involved in the study | |||||||||
| Isolated fungal species | Dogs | Cats | Parrots | G. pigs | Rabbits | Rats | Donkeys | Cows | Goats |
| M. canis | 16 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| M. gypseum | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| T. mentagrophytes | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| T. verrucosum | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| T. equinum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| F. oxysporum | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| F. chlamydosporum | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| niger | 4 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Penicillium spp. | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| A.alternata | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| M. pachydermatis | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| C. albicans | 4 | 2 | 0 | 1 | 1 | 2 | 0 | 1 | 0 |
| C.tropicalis | 9 | 12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| C.glabrata | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
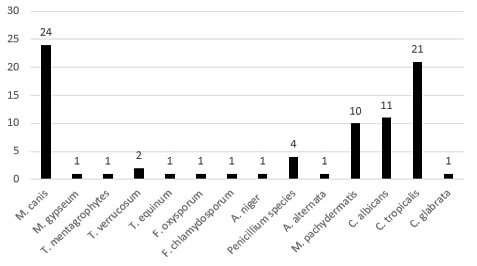
amined samples are represented in Figure (3). Concerning dermatophytes, about 30 species have been recovered. These included Microsporum canis [24], Microsporum gypseum [2], Trichophyton equinum [1], Trichophyton mentagrophytes [2], and Trichophyton verrucosum [1]. The isolated non-dermatophytes fungi included Malassezia pachydermates [10], Candida albicans [11], Candida tropicalis [21], Candida glabrata [1], Alternaria alternata [1], Penicillium species [4], Aspergillus niger [4], Fusarium chlamydosporum [1], Fusarium oxysporum [1], the rate of isolation of the different fungal species from different companion animals were illustrated in Table (1).
The results obtained from the direct microscopic examination, as shown in Table (2) were compared with fungal culture isolation. Out of the 600 samples, only 14 samples (2.33%) were positive by direct microscopic examination. Out of 14 microscopically positive samples, 12 samples (85.71%) were concordantly positive fungal cultures.
Table 2: Comparison between the results obtained from direct microscopic examination of collected samples and fungal isolation.
| Microscopic examination | |||
| Culture | Positive | Negative | Total |
| Positive | 12 | 73 | 85 |
| Negative | 2 | 513 | 515 |
| Total | 14 | 586 | 600 |
Cohen’s kappa correlation test has been performed on the results of both direct microscopic examinations and cultures. Based on proportion of observed agreement (po=0.87) and proportion of chance agreement (pe=0.83), k= 0.24. A poor correlation has been recorded between the two examination methods. The agreement plot is presented in Figure (4).
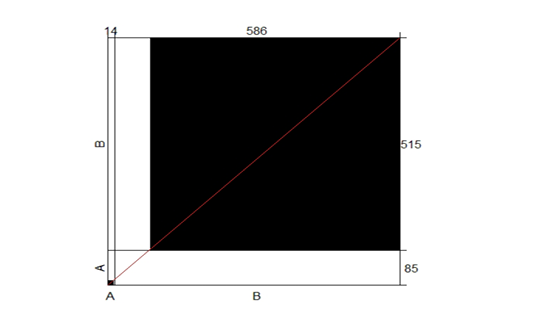
Figure 4: The agreement correlation of the two conventional laboratory testing methods used during the study, microscopic examination, and culture.
Strong agreement was observed between the two methods in case of negative results represented by the large black square concerning the large white rectangular, where a weak agreement was observed in case of positive results represented by the small black square concerning the small white rectangular. X-axis, A, and B are the positive and negative results obtained by microscopic examination and culture, respectively, Y-axis, A, and B are the positive and negative results obtained by microscopic examination and culture, respectively.
The number of isolates per sample was ideally 1 isolate per 1 sample; however, 5 samples showed mixed infection as follow, 3 mixed yeast infection, 1 of them was three Candida species C. albicans, C. tropicalis, C. glabrata, and the remaining 2 were mixed yeast and bacterial infection.
Results showed in Table (1) that the prevalence of fungal infections in the tested population was 14.16%. The most common fungal causes were dermatophytes that constitute 35% of the total fungal infections, and the most common dermatophyte species was M. canis that represented 30.5% of the total fungal infection and 80% of dermatophytes infection.
The different mold species either belong to the dermatophytes group or not were identified by using the reverse and the observe characters. The isolates were further identified using an examination of the different microscopic characters, including macroconidia, microconidia, chlamydospores, and conidial heads that were obtained using the microculture technique (Figures 5 and 6).
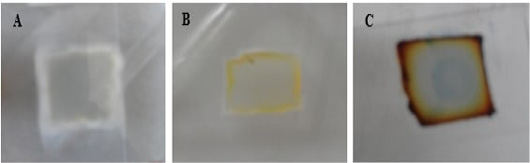
Figure 5: Macroscopic growth on SDA agar blocks of microculture set. A for T. mentagrophytes; B for M. canis and C for T. equinum.
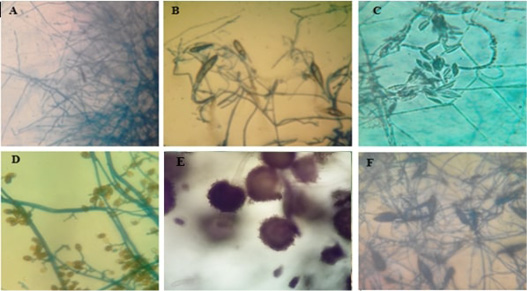
Figure 6: Microscopic pictures (using 40x high dry power lens) of different characteristic structures obtained from microculture technique.
A-Pencil shape macroconidia of T. verrucosum, B-Spindle shape macroconidia of M. gypseum. C- Crescentic shape macroconidia of F.oxisporum, D shows club shape macroconidia with both horizontal and longitudinal septa of A. alternata. E-Conidial head of A.niger. F-Rough surface spindle shape of macroconidia of M.canis.
The illustrations in Figure (7) demonstrated the different yeast species identified by direct microscopic examination using Grams’ technique. The isolates showed large-size grams’ positive budding yeast cells besides two very characteristic snowman/bowling pin/footprints pattern in the case of M. pachydermatis. The isolates were further identified using the urease test in association with candida chromogenic agar where C. albicans gave green colored colonies, C. tropicalis violet color, and C. glabrata beige color.
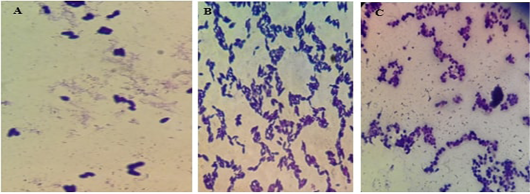
Figure 7: Microscopic pictures using the oil immersion lens of different yeast isolates stained with gram’s technique. A for M. pachydermatis showing the characteristic bowling pins pattern, B for C. tropicalis and C for C. albicans.
DISCUSSION
The conducted analysis was wholly focused on the samples from animals with cutaneous lesions and showed that in 14.16% of examined animals, different fungal elements were isolated and identified. A wide range of fungal agents belonging to basidiomycetous and ascomycetous yeast, as Malassezia and Candida spp, hyaline mycelial molds as Aspergillus, Penicillium, and Fusarium, dematiaceous mycelial molds as Alternaria spp, as well as typical dermatophytes, were isolated and clearly identified. Out of the 600 samples incorporated in the current study, yeasts were isolated in 50.6% of the positive cultures (Malassezia pachydermatis in 11.7% and Candida spp. in 38.8%), while dermatophytes were demonstrated in 35.3% of the positive cultures. Cats and other companion animals such as rabbits and guinea pigs are considered as dermatophytes’ carriers (Gnat et al., 2019; Nenoff et al., 2014). Moreover, the dermatophytes group is considered a contagious fungal agents as it is transmitted through different animal’s equipment and fomites, like brushes, towels, collars, and peds. Dermatophytes characterized by a highly resistant spores which can remain active in in the environment for up to one year. Among all cutaneous mycoses, dermatophytosis is a zoonotic disease and can be transmitted from animals to humans and vice versa in case of zoophilic dermatophytes spp. Clinical cases of dermatophytosis co-occurring in animals and its owner were described and reported (Kursa et al., 2011; Van Rooij et al., 2012; Grills et al., 2007).
In contrast to previous studies from different parts of the world, Iran (Khosravi, 1996), Northern Italy (Proverbio et al., 2014), Mexico (Chavez et al., 2000), India (Murmu et al., 2015), the present study showed a higher prevalence of dermatomycosis generally and dermatophytosis specifically in dogs than cats.
During the current investigations, various hyaline molds such as Aspergillus, Penicillium, or Fusarium were obtained. However, growth of those non-dermatophytes hyaline molds while culturing animal cutaneous samples, could be considered as contaminating fungal agents because their spores are present extensively in the air. They are characterized by wide environmental and distribution and deposition on animals’ hair coat (Mazur et al., 2018). However, a series of successive culturing of the same sample for several times and obtaining the same isolation results would suggest potential keratinophilic abilities for those isolates and strains.
During the period at which the current study was conducted, Alternaria was one of those mycelial fungi isolated from cases of cutaneous infection. The growth of dematiaceous fungi colonies is considered as a typical saprophytic fungal contamination of growing cultures as they are severely distributed in the environment (Lee et al., 2015; Meason-Smith et al., 2015; Dąbrowska et al., 2018; Lee et al., 2015). However, in veterinary medicine, dermatomycoses due to molds such as Alternaria alternata, have become an emerging clinical problem. In the current study, only one isolate of Alternaria was obtained, representing 1.2% of positive cultures. Despite Alternaria world-wide distribution and presence, reports about Alternaria dermatomycoses were rare. In 2013, spores of Alternaria proliferating in the skin scrapings were reported and isolated as a sole causative agent of fungal dermatitis in horses and dogs (Dworecka-Kaszak, 2013; Tyczkowska-Sieroń and Głowacka, 2013).
The current analysis of the obtained samples did not reveal any positive results for dimorphic fungi. However, previous studies has demonstrated that histoplasmosis reported in Egypt (Soliman et al., 1991) and cryptococcosis in Egyptian animals and wild birds (Refai et al., 2017), while the presented study showed that they are usually not found in companion animals.
In addition to the isolated dermatophytes molds and non-dermatophytes molds, a non-lipid dependent species of Malassezia genus was isolated. These yeasts were reported to cause infections in different regions of the body of both humans and dogs (Findley et al., 2013; Meason-Smith et al., 2015). Fundamentally, the lipophilic Malassezia species colonized only on human skin, while M. pachydermatis restricted to animal skin, especially dogs (Pier et al., 2000). Other Malassezia species, like M. globosa or M. sympodialis, and lipid-dependent strains of M. pachydermatis could be obtained from healthy cats and dermatomycotic lesions in dogs (Pier et al., 2000; Bond et al., 1997; Cafarchia et al., 2005; Puig et al., 2017), but they were unculturable in the current conducted study. Although, these yeasts are opportunistic in nature, it may turn pathogenic with normal cutaneous microbiota disruptions, flea bites-dermatitis, atopy, and uncontrolled corticosteroids application (Pier et al., 2000; Cafarchia et al., 2005; Bajwa, 2017; Nardoni et al., 2007).
In cats, Malassezia infections were associating other predisposing disorders such as retroviral infections, immunodeficiency, and extensive antibiotic therapy (Pier et al., 2000). M. pachydermatis, were also reported in wild and exotic animals (Pier et al., 2000). In the current study, M. pachydermatis was obtained from over 11.7% of the positive fungal cultures. These opportunistic yeasts are of increasing importance in the dermatomycotic cases (Chen et al., 2017; Batra et al., 2005; Findley et al., 2013; Faergemann, 2002). Cases of systemic fungemia in humans have been associated with M. pachydermatis, for which dogs are the transmitters of infection. In other cases, the sources of human infections have been derived from companion dogs (Saridomichelakis et al., 2007; Morris et al., 2005).
During the one year period at which the current study was conducted, various Candida spp were identified, mainly C. albicans in 12.9% of positive fungal cultures, C. tropicalis and C. glabrata were isolated also. Through the study, the number of Candida isolates obtained from companion animals was incomparable with the number of Malassezia isolates. The involvement of different yeast fungal causes in companion animal’s cutaneous infection indicates that fungi belong to different yeast genus and species are important factors of dermatomycoses in various companion animal species.
The current cross-sectional study revealed a wide range of fungal causes, either yeast or mold that has been isolated and identified from various dermatological affections in companion animals from the Greater Cairo Area, Egypt. Previous studies have been conducted throughout different geographical areas of Egypt as Behera (Haggag et al., 2017), Ismailia, and Port-said (Aboueishsa and El-Mahallawy, 2013), but there were not enough studies performed to configure the epidemiological occurrences of different fungal elements in GCA, Egypt. Reaching a clear panel of the most common fungal causes in this area, and the different frequencies of various causes will lead to further future investigations and prospective studies. Further studies would be directed toward completing the whole epidemiological model by performing an antifungal sensitivity test of each isolate to have an overall antifungal activity and antifungal resistant ability of different fungal elements in this area. Also, as the obtained isolates, consider hot virulent strain isolated from active clinical cases, from this study were identified and preserved, future incorporation in vaccine production trials or rapid diagnostic kit developing, which will consider true advances in the field of diagnosis of dermatomycosis.
CONCLUSION
Dogs showed a higher prevalence of dermatomycosis than other companion animals, and a weak agreement correlation has been revealed between microscopic examinations and cultures as conventional methods of laboratory diagnosis in case of cutaneous fungal infections.
Human and animal companionships has been increased severely in the past few decades, the number and species of domesticated animals also increase day after day. Therefore, a screening of companion animal diseases, especially those of zoonotic importance, becomes a mandatory scientific need due to the impact of such studies and its public health importance in the era of «one world, one health» launched by the world health organization (WHO) in the current millennium.
ACKNOWLEDGMENT
We want to acknowledge Professor Ahmed Samir (Microbiology Department, Faculty of Veterinary Medicine, Cairo University) for the collaboration during the different stages of the study, and we would like to thank all general practitioner veterinarians in GCA, Egypt, due to the help in sample collection stage.
CONFLICT OF INTEREST
Authors have no financial or personal relationship with other people or organizations that could lead to unethical bias affect the content of this article.
Source of Funding
This is a self-funded study.
Ethical Approval
The approval from the Institutional Animal Ethics Committee to carry out this study was not required as no invasive technique was used against animals, and all samples are collected from animals suffering from cutaneous lesions permitted to veterinary clinics or veterinary laboratories.
authors contribution
The authors; Hassan A. Hassanien, Rafik H. Sayed and Heidy Abo Elyazeed contributed to the design and implantation of the research, to the analysis of results and to the writing of the manuscript.
REFERENCES