Advances in Animal and Veterinary Sciences
Research Article
Determination of Median Lethal Dose of Zinc chloride in Wistar Rat
Siva Kumar Tekuri1, Purusotham Bassaiahgari1,2, Yuvaranjani Gali1, 3, Shobha Rani Amuru1,4, Neeraja Pabbaraju1*
1Department of Zoology, Sri Venkateswara University, Tirupati-517502, Andhra Pradesh, India; 2Department of Zoology PVKN Govt. College (Autonomous), Chittoor-517002, Andhra Pradesh, India; 3Department of Zoology, Govt. Jr. College, Irala, Chittoor-517130, Andhra Pradesh, India; 4Department of Biosciences & Sericulture, Sri Padmavathi Mahila Visvavidyalayam, Tirupati-517502, Andhra Pradesh, India.
Abstract | Zinc is one of the most abounded trace elements in the body following iron. Zinc when supplemented in a trace amount participates as a catalytic cofactor of enzymes and regulates various metabolic functions in the body. Excess supplementation of any trace element disturbs the metabolic functions of organs and leads to toxicity. The aim of the work was taken to find out the median lethal dose of zinc chloride (ZnCl2) to albino rats via intraperitoneal (IP) route. A single dose of ZnCl2 was dissolved in distilled water (Milli-Q) and administered intraperitoneally at the concentrations of 10, 20, 30, 40, 50, 60, 70, 80, 90, and 100 mg/ kg body weight of the experimental animals. Experimental units were observed every 3 hrs before dose administration and later after 6 hrs, 12hrs, 24 hrs and 48hrs for the development of any abnormal behaviors and toxicity symptoms. After 48 hrs, mortalities were estimated in each group in correlation to the total susceptible population. The obtained results were evaluated by the Statistical Probit Analysis Method. The results revealed that the lethal dose 50 (LD50) value within 48 hrs in Albino rats was 57.348 mg/kg b.w. The identification and evaluation of the LD50 against zinc chloride were essential for understanding zinc toxicity because it has been commercially utilized in the form of inorganic zinc salt as a therapeutic agent, dry cell batteries, and for household purpose, thus information about the toxic impacts on ZnCl2 is useful for experimental or toxicological approaches. The in-vitro LD50 evaluations of target chemicals in Wister rats were highly associated with zinc toxicity-related physiological disorders perceptive and therapy.
Keywords | Zinc chloride, Trace element, Median lethal dose, LD50, Mortality
Received | December 07, 2020; Accepted | December 17, 2020; Published | January 15, 2021
*Correspondence | Neeraja Pabbaraju, Department of Zoology, Sri Venkateswara University, Tirupati-517502, Andhra Pradesh, India; Email: pneeraja.bio2015@gmail.com
Citation | Tekuri SK, Bassaiahgari P, Gali Y, Amuru SR, Pabbaraju N (2021). Determination of median lethal dose of zinc chloride in wistar rat. Adv. Anim. Vet. Sci. 9(3): 393-399.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.3.393.399
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Pabbaraju et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Trace elements such as zinc, copper, selenium, and iron are essential for living organisms. At the obligatory level, each trace element has a specific functional role, like metabolic functions. Significant levels of trace elements are constructive to the living forms that possess various metabolic activities (Bhattacharya et al., 2016). An insufficient amount of trace elements lead to various malfunctions like, abnormal body functions, malnutrition, and deviated growth and development of the organisms. Exposures at excess levels of trace elements might lead to accumulating in the body fluids that predominantly disturb the body functions and contribute to toxicity. Generally, exposure to significant levels of trace element is occurring through the food chain, inhalation, drinking, or eating trace elements in the food. Although several environmental pollutants like lead, cadmium, arsenic, and mercury can bind strongly with soil and sediments that tend to persist in nature (Jaishankar et al., 2014). They imitate normal functional trace elements and are strongly associated with the antioxidant system, deoxyribonucleic acid (DNA), proteins, and endogenous steroid hormone receptors (Tchounwou et al., 2012).
Zinc is well known ubiquitous trace element, which is an indispensable component for the growth and development of microorganisms, plants and animals (Jurowski et al., 2014). It plays an important role in the formation of bio-molecules, acts as a catalyst, co-catalyst for enzymes, and scavenging the reactive oxygen species (ROS) during stresses and diseased conditions. Studies have moderately defined that whether these functions are hierarchical in terms of zinc utilization. However, zinc insufficiency or excess can moderate a cascade of metabolic processes, which shows adverse health effects (Nriagu, 2011).
Overall, the determination of the toxicity of zinc is a very important aspect in the clinical and preclinical experiments, which signifies how much amount of zinc is safe for health and pharmacokinetics importance. However, determination of the toxic levels of zinc using the median lethal dose (LD50) methods are poorly evaluated; therefore, urgent scrutiny is required to determine the unsafe levels of zinc uptake includes bioavailability nature. Hence, the present study has been attempted to determine the median lethal dose of zinc chloride (ZnCl2) via intraperitoneal route and acute systemic changes associated with lethal and sub lethal doses in rats. Therefore, the predicted lethal dose data are potential for experimental, clinical and preclinical aspects toward understanding the toxicity of ZnCl2 for toxicologists.
MATERIALS AND METHODS
Test chemical
The Zinc chloride (ZnCl2) hydrate chemical (98% pure, solid) was obtained from Molychem Laboratory, Mumbai, India.
Animals and experimental design
Healthy Wister strains Albino rats of the same age group (100±10days) and weight 200±10g were selected as experimental animals for the present study. The rats were procured from the Indian Institute of Science, Bangalore, India. Before experimentation, the animals were acclimatized to laboratory conditions. Rats were housed in polypropylene cages lined with clean paddy husk, and provided with filtered tap water and rat chow (obtained from the Saidurga agencies Animal Feed, Bangalore, India) ad libitum in a laboratory conditioned environment (34±20 C). The room where the rats were kept provided with a lighting regimen based on a 12-hrs light and 12-hrs darkness. To ascertain LD50, six groups of Albino rats, each group comprising of 10 animals were taken before dose administration, animals were fasted for 6 hrs. The experiments were carried out in accordance with the guidelines of the Institutional Animal Ethical Committee, Sri Venkateswara University, Tirupati, India (Resolution No. 10/(i)/a/CPCSEA/ IAEC/SVU/ZOOL/PN/Dt. July 08, 2012).
Dosage preparation and administration
Zinc chloride was dissolved in distilled water (Milli-Q) and injected intraperitoneal to the rats at different concentrations through a syringe (1ml). The volume of the dose depends on the size and weight of the animals. The calculations were done using the Origin Pro 8 software.
RESULTS
Behavior signs during the experiment
Required levels of zinc did not show any physiological abnormalities or, toxic signs in the animals. Zinc affects animals when their level exceeds the normal and required levels in body fluids and tissues and becomes toxic. In the present study, the prior administrated dose 10 mg/kg b.w did not have any toxic signs on the central nervous system of rats. But the doses of 20-90 mg/ kg b.w were administered to the animals; abnormal CNS stimulations and behavioral abnormalities were observed. The animals exhibited behaviors like jumping and leaping movements in cages, scratching at the test dose injected place. At the higher doses, i.e., 80, 90 and 100 mg / kg b.w animals exhibited hemorrhage and bleeding in the tail ends. Finally abdomen frequently became inflated and the animals were not able to walk normally, exhibiting slow, writhing and caricature movements of the neck and tail, then predominantly underwent to gasping and death.
LD50 calculation through the conversion of percent mortality to probit
The LD50 of zinc chloride has been calculated based on the analysis method of probit by the conversion of mortality percentages to probit as revealed, by Finney, (1971). The data on the different dosages of zinc chloride administered to animals were shown in Table 1. Critical observation of the final results indicated that the mortality rates of animals were observed based on the dosages of the selected chemical (zinc chloride). Before administration of zinc (10 mg/ kg), no mortality was observed in rats. At 20 mg/ kg dose, about 10% of animals died. In parallel mode, 20% mortalities were observed at 30mg/kg dosage, 30% mortalities were observed at the dosage of 40 mg/kg. Then consequently 40%, 50%, 60%, 70%, 80%, and 90% mortality rates were observed at concentrations of 50, 60, 70, 80, 90, and 100 mg/kg, respectively. When the dose concentrations were increased, the mortality rates were also increased. It was also evident that 50% mortality was observed at the concentration of 60 mg/kg B.w. The obtained data from dose to percent mortality were processed to derive 50% mortality and the graph gave curvilinear sigmoid or S- shaped curve and finally, the LD50 was found to be 57. 348mg/kg b.w as presented in Figure-1. The percent mortality values were converted into probit units and plotted against log concentration (Figure-2). By using this method, a sigmoid growth curve was observed with 0.97 adjacent R square value (Figure-1). Whereas, the linear regression analysis was performed between the Log concentrations with probit mortality using linear regression fitting method also showed 0.99 adjacent R square value with straight line instead of sigmoid curve (Figure-2). The relationship was found to be linear obeying the principles of dose-response studies.
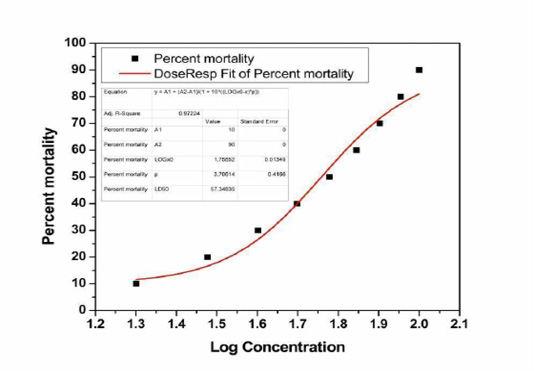
Figure 1: Sigmoid “Graded Response” curve showing the relation between the log concentrations of Zinc chloride and percent mortality of the albino rat.
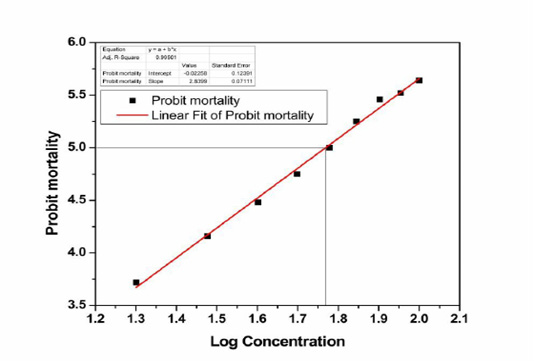
Figure 2: Graph showing straight-line relation between the log concentrations of Zinc chloride and probit mortality of the albino rat.
DISCUSSION
Zinc is one of the environmental pollutants and omnipresent trace elements in the environment. Excess levels of zinc supplementation contribute to zinc accumulation in the body fluids causing interruption of metabolic activities and lead to toxicity of the living organisms. The intemperance levels of zinc salts enter into the body by inhalation, ingestion or through the skin and induce irritation of the respiratory and digestive system, dental deterioration, and ulceration of the skin. Zinc fumes cause fever, chills, nausea, vomiting, muscular aches and weakness. The main sources of zinc toxicity are zinc salts that are widely used as a protective coating of other metals, in die casting, in industry, and for alloys. Inorganic zinc compounds have various applications like, automotive equipment, storage, dry cell batteries, dental, medical and household applications. Organo-zinc compounds are used as fungicides, topical antibiotics, lubricants, food supplements, additives, medicines, disinfectants, antiseptic, deodorant preparations, and in dental cement. Drinking water can be considered as a serious source for introducing high and excess levels of zinc levels into the body, these high levels might be attributed to the flowing of water through galvanized zinc protective coating copper or plastic pipes. Zinc salts like zinc chloride, acetate, sulfate and phosphide compounds are commercially used as herbicides to control the growth of moss on patios, walkways, lawns and structures for different applications. Commercial zinc salts chemically are strong eye irritants and contribute to, pain and erythema which may be complicated with several manifestations like by corneal ulceration, edema, burns, iritis, hyperemia, hemorrhaging, bullous keratopathy, glaucoma, cataract formation, discrete grey spots on the lens, lacrimation, significant reduction in visual acuity with conjunctival hemorrhage and inflammations. Dilute solutions of zinc chloride (<1%) are non-irritating and have been used in the medicinal fields as eye drops, a 20% solution of zinc sulfate has been used to several eye problems, but contributed to the formation of white flecks on the lens of the eye and this practice has been discontinued (Plum et al., 2010; Nriagu, 2011).
Over exposure to zinc oxide leads to skin-irritating, vomiting, nausea, seizure and fatigue. Inhalation of zinc oxide responsible for respiration (breathing) problems, consequently leads to damage to the respiratory organ (lung), as well as, damages the liver, kidney organs. Zinc phosphide well-known rodenticide and widely used as an insecticide in the agricultural fields (Knight, 2013). Accidental ingestion of zinc phosphide reacts with water and gastric juice to liberate phosphine gas, which can enter into the blood stream and contribute to CNS depression, irritation of the lungs, damage to the liver, kidney, heart, and CNS, stomach pains, and diarrhea in mild zinc phosphide toxicity (Abdel et al., 2011), vomiting, chest tightness, excitement, unconsciousness, coma and death occur due to pulmonary edema, heart failure and liver damage in severe zinc phosphide toxicity.
Table 1: Mortality Wister albino rats exposed to different concentrations of Zinc chloride at 48 h (Mortality expressed in both per cent and probit kill number of animals exposed to each time is 10).
| S.No | Concentration of Zinc chloride (mg/l) | Log Concentration | Number of Rats | Percent mortality | Probit mortality | |
| Exposed | Dead | |||||
| 1 | 10 | 0 | 10 | 0 | 0 | 0 |
| 2 | 20 | 1.301 | 10 | 1 | 10 | 3.72 |
| 3 | 30 | 1.477 | 10 | 2 | 20 | 4.16 |
| 4 | 40 | 1.602 | 10 | 3 | 30 | 4.48 |
| 5 | 50 | 1.698 | 10 | 4 | 40 | 4.75 |
| 6 | 60 | 1.778 | 10 | 5 | 50 | 5.00 |
| 7 | 70 | 1.845 | 10 | 6 | 60 | 5.25 |
| 8 | 80 | 1.903 | 10 | 7 | 70 | 5.46 |
| 9 | 90 | 1.954 | 10 | 8 | 80 | 5.52 |
| 10 | 100 | 2.000 | 10 | 9 | 90 | 5.64 |
Zinc chloride is an inorganic compound used as a catalyst and preservative agent for food, soldering fluxes, electro deposition, antiseptic preparations, textiles (mordant’s, mercerizing agents), adhesives, dental cements, and medicine (astringent) (Bautista-Gallego et al., 2011; Kadam et al., 2015; Toledano et al., 2017). Occupational exposure to zinc chloride through inhalation following the military use of “smoke bombs” has resulted in interstitial edema, interstitial fibrosis, pneumonitis, bronchial mucosal edema, ulceration and even death under extreme exposure conditions. These effects are possibly attributable to the hygroscopic and astringent nature of the zinc chloride particles (Plum et al., 2010; Nriagu, 2011). Consequently the prediction of toxicity of zinc chloride plays a prominent role to understand in the evaluation of toxicity levels in organisms and how much zinc salt can cause toxicity in different organisms.
Therefore, zinc toxicity determination is very important to measure the acute and chronic toxicity of a substance, food poisoning and accidental domestic poisoning. The types of toxicity tests which are routinely performed by pharmaceutical manufacturers in the investigation of a new drug involve acute, sub-acute and chronic toxicity. Acute toxicity is involved in the estimation of the LD50 that represented the dose which has proved to be lethal to 50% of the experimentally tested animals (Raj et al., 2013; Siva Kumar et al., 2017).
The present work mainly focused on the evaluation of lethal dose of zinc chloride in male Wister rats by intraperitoneal route. Intraperitoneal administration of 10 mg/kg b.w to rat’s showed no behavioral and physiological abnormalities; therefore this is considered to be a safe level (NOAEL). However, animals were treated with different concentrations from 40 to 80 mg/kg b.w. doses showed a sequence of toxic signs and behavioral abnormalities, but the earliest doses of 20 and 30 mg/kg b.w. showed mild toxic signs. The graphic illustration of mortality rates versus log concentration and probit mortality versus log concentration (Table-1) showed a distinctive sigmoid curve (Figure-2) and a straight linear curve (Figure-2) correspondingly which were in superior concurrence with the principle of probit analysis (Finney, 1971).
The present obtained data from dose to percent mortality rates were processed to derive 50% mortality and the graph gave curvilinear sigmoid or S-shaped curve, and LD50 was found to be 57.348mg/kg b.w, this was represented in Figure-1. The 50% mortalities were found to be 60mg/kg b.w dose, but when probit mortality was calculated for 60 mg/kg b.w to log. The value was found to be 1.7526 log concentration by applying the formula 5.0-Intercept/Slope. When 1.7526 log concentration values were found at dose concentration (Table 1) between the 50-60mg/kg. The log concentration and mortality rates data processed in origin software, the accurate LD50 was found to be 57.348mg/kg b.w at the 1.7526 log concentration. So the exact lethal 50% mortality of ZnCl2 may fall in between 57.348 mg/kg b.w I.P in rats. This was proved by the obtained sigmoid or S-shaped curve plotted percent kill and log concentration values on the y and x-axis respectively. The straight-line also support that the LD50 of ZnCl2 is 57.348 mg/ kg B.w.
Acute toxicity studies on different zinc salts (Zinc nitrate, Zinc acetate dehydrate, and Zinc sulfate) via intraperitoneal administration within 48 hrs exposure periods in albino rats and mice, showed different LD50 values. Zinc nitrate administered rats and mice showed that the effective LD50 values were 133 mg/kg b.w, and 110 mg/kg b.w respectively. Zinc sulfate and Zinc acetate treated rats and mice showed effective LD50 values 200 mg/kg b.w, and 316 mg/kg b.w, 162mg/kg b.w and 108 mg/kg b.w LD50 values respectively (Domingo et al., 1988). Wang et al. (2008) evaluated the toxicity test of ZnO nanoparticles in exposed mice with nanometers of ZnO (Ø 20 nm), ZnO (Ø 120 nm) ZnO (Ø 20 nm) and showed that the LD50 was greater than 5000mg/kg b.w, although ZnO (Ø 120 nm) administered rats represents greater than 2000 to less than 5000mg/kg b.w LD50 value (Wang et al., 2008). Jing-Hui and Marsh, (1988) conducted toxicity evaluation of Zinc phosphide in household mice and albino laboratory mice of both male and female sexes. The LD50 for wild house mice (sexes combined) was 32.68 mg/kg and for albino mice was 53.34. Finally, it was concluded that wild mice are more susceptible to zinc phosphide compared with albino mice (Jing-Hui and Marsh, 1988). Zinc chloride toxicity evaluation in Swiss albino mice for 96-hrs LD50 obtained as 1230mg/kg b.w via oral administration (Yadav and Trivedi, 2016).
Zinc-related nanoparticles (Zn NPS) have been increasingly used nowadays in the traditional industries, like paints, electrically conducting materials, ceramic, chemical daily expenses, catalysts and other fields. Due to their special properties, the production and application processes of Zn Nps were increasingly in the nanotechnology industrialization (Dastjerdi and Montazer, 2010; Wang et al., 2017). Zinc oxide nanoparticles have acute toxicological effects in mice after intratracheal instillation of 200, 400, 800 μg/kg, Wang et al. (2017) reported that ZnO NPs were responsible for the elevated levels of total cell number, total protein, and hydroxyproline content and loss of body weight. Inflammatory, hyperplastic histological changes in the lungs were also observed. This study also reported that the LD50 in the intratracheal instillation of ZnO NPs (48 nm) was 493.85 μg/kg body weight (Wang et al., 2017). ZnO NPs (5.6 mg/kg, intraperitonial) induced increased production of pro-inflammatory cytokines in the serum and the brain of mice (Tian et al., 2015). ZnO NPs accumulation causes renal damage in rats by evidently increases in the levels of taurine, lactate, acetate, creatine, phosphocholine, trimethylamine-N-oxide, α-glucose, and 3-D-hydroxybutyrate, as well as decreases in lipid, succinate, citrate, α- ketoglutarate, hippurate and 4-hydroxyphenylacetic acid in urine after ZnO NPs (1000mg/kg) intragastrically treatment daily for successive 14 days. Tubular epithelial cell necrosis observed kidney histopathological examination at 1000mg/k (Yan et al., 2012). Adamcakova-Dodd et al. (2014) also evaluated the ZnO NPs sub-acute and sub-chronic toxicity in murine inhalation models (mice) and reported that ZnO NPs (3.5 mg/m3, 4 hr/day) for 2 (sub-acute) or 13 (sub-chronic) weeks causes increase of macrophages in BAL (Bronchoalveolar lavage) fluid and a moderate increase in IL-12(p40) and MIP-1α, but no other inflammatory or toxic responses were observed (Adamcakova-Dodd et al., 2014).
Zinc deficiency leads to sterility in animals by improper production of hormones; normal optimized levels on the other hand can enhance sperm production and ovum release in both genders. In contrast, excessive accumulation of zinc content in the body fluids causes reproductive toxicity in animals and humans. Turgut et al. (2003) reported that, 2.5 g Zn/100mL drinking water to rats for 3 weeks exhibited negative reproductive results. They noticed degenerative changes in testis histopathological examination, including spermatic arrest, degeneration of seminiferous tubules, and fibrosis in the interstitial tissue. Sperm count and sperm motility alterations occurred in high dose zinc sulfate treated rats (Turgut et al., 2003). The excessive ZnCl2 accumulation during pregnancy or before and during lactation time caused developmental toxicity to offspring and mothers (Johnson et al., 2011). Zinc-induced copper deficiency resulting in neutropenia, anemia, and severe progressive peripheral neuropathy (Willis et al., 2005).
Toxicity determination studies were also well reported in the field of aquaculture. Several researchers evaluated the toxic nature of zinc salts in fish. Gul et al. (2009) conducted the acute toxicity studies of zinc sulfate on widely distributed tropical fishes; LC50 value was found to be 30.826 mg/l for 96 hours (Gul et al., 2009). The zinc sulfate LC50 value in air-breathing fresh water fish, Channa punctatus via the I.P route for 96 hrs was found to be 16.5mg/L (Nikam, 2012). Pourkhabbaz et al. (2011) conducted acute toxicity studies of ZnSO4 on the eastern mosquito fish -Gambusia holbrooki in three different water hardness concentrations (25, 125, and 350 mg L CaCO3) like soft, hard, and very hard water at different exposure times (24, 48, 72, and 96 hours). In the soft water, the recorded LC50 values at 24, 48, 72, and 96 hours of exposure were 1.58, 0.67, 0.52 and 0.46 mg L-1, respectively, in the hard water 66.05, 58.38, 52.3, and 48.1 mg L-1, respectively, and in the very hard 135.8, 126.4, 122.9, and 121.6 mg L-1, respectively (Pourkhabbaz et al., 2011). The lethal concentration of 50 was higher in soft water when compared to hard and very hard water concentrations (from 25 to 350mgL-1 CaCo3), which indicates that an increase in water hardness substantially reduces the toxicity of Zn to G.holbrooki for 96 hrs of exposure.
Several studies also revealed that excess accumulation of zinc salts in water bodies (rivers, ponds) accounted for the histological, morphological and physiological changes in the aquatic organism. Loganatha et al. (2006) reported histological changes in the brain and liver of Labeo rohita (Ham.) exposed to sublethal concentrations of 5 and 10 ppm during 5 and 15 days time interval. The sub-lethal concentration chosen based on the 96 hrs LC50 zinc was found to be 156 ppm in Labeo rohita. A dose of 5 ppm caused the swelling of pyramidal cells with binucleate nuclei, whereas at the 10 ppm exposure, severe necrosis of neuronal cells of the cerebrum was evident, indicating loss of nissel substances. The liver of Labeo rohita at the 5 ppm exposure showed severe necrosis, hemorrhage, distended sinusoids with minor vacuolation. At 10 ppm exposure, pyknotic nuclei were more prominent with indistinct single cells (Loganatha et al., 2006). Abdel-Warith et al. (2011) reported the histological effects of zinc chloride on liver of Nile tilapia, Oreochromis niloticus and they stated that short term (one week) exposed aquarium fishes exhibit prominent histological changes, while regenerative responses were noted in fish exposed over the long-term (4 weeks) period. They also reported that the ZnCl2 LC50 96 hrs for was to be 8mg/L.
The median lethal concentration (LC50) of Zinc Nanoparticles (Zn Nps) on Oreochromis niloticus and Tilapia zillii after 24, 48 and 96 hrs exposure times exhibit different LC50 values. 24, 48 and 96 hrs LC50 values in Oreochromis niloticus were 7.48, 6.9, and 5.5 mg/L respectively; however, in Tilapia zillii lethal toxic values were 7.6, 7.0, and 5.6 mg/L respectively. Fish exposed to 2000 µg L Zn Nps caused severe oxidative stress on brain tissue and lead to the decrement of antioxidant enzymes (GSH, tGSH, SOD, CAT, GPx, GR and GST) and increased levels of MDA (Saddick et al., 2015). Abdel-Khalek et al. (2015) also used Nile tilapia; Oreochromis niloticus to evaluate the impact of zinc Nanoparticles on aquatic ecosystems, they noticed that the 96 hr LC50 of zinc bulk particles (BPs) and Zn NPs on O. niloticus were 1.36 and 0.18g/l, respectively. When the exposure of 96 hr 1/2 LC50 of zinc bulk particles (0.68g/l) and Zn NPs (0.09g/l) concentrations were standard for 7, 14 and 28 days, caused alteration in lipid, protein, globulin contents, liver marker enzymes and concomitant decrement of antioxidant enzymes.
CONCLUSION
The determination of the median lethal dose of 50 (LD50) of Zinc chloride in albino rat provided useful pharmacological and toxicological information relating to human protection. Accordingly zinc finds a wide range of commercial uses, and prolonged exposure of human beings to zinc compounds poses high risk of occupational hazards. Therefore the determination of toxicity tests is essential to find out what concentration of zinc affects physiological and histological changes in animals as well as humans. The present study represents acute zinc chloride toxicity in male albino rats and determination of LD50 of 48 hrs exposure via intraperitoneal (IP) route administration. The most accurate estimate of the acute LD50 in rats is 57.348 mg/kg.
Acknowledgement
T. Siva Kumar (SRF) grateful to University Grants Commission (UGC), New Delhi, for awarding BSR-RFSMS/SRF research fellowship.
conflict of interest
The authors declare no conflict of interest.
Authors contribution
All authors contributed significantly to the work presented in this manuscript. PN: conceived the study and designed the experiments, supervised the study and wrote part of the manuscript and typos correction. SK, PB, and YG: carry out experimental work. SK: drafted the manuscript, done statistical data analysis, and graphical representation. PB, YG: participated in interpretation of manuscript and editing. SR: helped in statistical analysis of data, graphical representation in origin and manuscript typos errors checking and editing.
References





