Advances in Animal and Veterinary Sciences
Research Article
Antifungal Activity of Natural Essential Oils against Molds and Yeasts Associated with Respiratory Problems in Broiler Chickens
Ahmed Hussein Abed1, Ismail Abdel-Hafeez Radwan1, Moshira Mohammed Abd El-Aziz2, Ahmed Ali3*
1Department of Bacteriology, Mycology, and Immunology, Faculty of Veterinary Medicine, Beni-Suef University, Beni-Suef 62511, Egypt; 2Directorate of Veterinary Medicine, Beni-Suef 62511, Egypt; 3Poultry Diseases Department, Faculty of Veterinary Medicine, Beni-Suef University, Beni-Suef 62511, Egypt.
Abstract | In recent years, essential oils (EOs) have been investigated for their antifungal activity which could predict therapeutic benefits for fungal diseases as an excellent alternative for the chemical medication. In this study, the antifungal activity of different concentrations (0.05, 0.1, 0.2, 0.3, and 0.5%) of thyme, anise, and cinnamon EOs was performed against of 51 fungal isolates (36 molds and 15 yeasts) recovered from broiler chickens using the agar dilution method. Thyme oil inhibited the growth of all tested isolates at concentrations of 0.5, 0.3, and 0.2% while concentration of 0.1% inhibited the growth of 100% of A. niger and C. albicans isolates as well as 40 and 81.2% of A. flavus and A. fumigatus isolates, respectively. Cinnamon oil inhibited the growth of all tested isolates except at 0.05% concentration which inhibited only 40, 20, 62.5 and 86.7% of A. niger, A. flavus, A. fumigatus, and C. albicans isolates, respectively. The anise oil had the lowest activity and 0.5% concentration only inhibited completely the fungal growth. Additionally, 8 isolates were selected and subjected to the Internal Transcribed Spacer (ITS) gene-based PCR, sequencing, and comparative sequence analyses. PCR results confirmed the morphotypic identification of fungal isolates and all the selected isolates possessed the ITS gene. Phylogenetic and sequence analysis showed that A. niger, A. fumigatus, and C. albicans avian isolates are closely related to human fungal isolates including those causing respiratory affection and recently reported emerging azole-resistant fungi. However, A. flavus isolates were related to toxic aflatoxins producing fungi. The current study concluded that cinnamon and thyme EOs had strong antifungal activity indicating their potential usefulness for antifungal preparations. Moreover, the ITS gene sequencing is a robust technique for the identification of fungi on the species and relatively on the strain levels.
Keywords | Aspergillus spp., C. albicans, Broiler chickens, Essential oils, Thyme, Cinnamon, ITS gene
Received | September 08, 2020; Accepted | December 03, 2020; Published | January 15, 2021
*Correspondence | Ahmed Ali, Poultry Diseases Department, Faculty of Veterinary Medicine, Beni-Suef University, Beni-Suef 62511, Egypt; Email: ahmed.ali1@vet.bsu.edu.eg
Citation | Abed AH, Radwan IA, El-Aziz MMA, Ali A (2021). Antifungal activity of natural essential oils against molds and yeasts associated with respiratory problems in broiler chickens. Adv. Anim. Vet. Sci. 9(3): 348-355.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.3.348.355
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Abed et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
The poultry industry is facing various constraints including infectious diseases. In Egypt, the poultry industry is one of the main agricultural industries reaching more than 20 billion LE investments (Radwan et al., 2018c). Fungal infections are frequently associated with morbidity and mortality in birds. Few fungal species are common pathogens in avian species especially Aspergillus spp. The cause of aspergillosis is mainly Aspergillus fumigatus. Other species like A. flavus, A. niger, A. nidulans, and A. terreus may also be isolated from avian cases of aspergillosis (sometimes in mixed infections) but much less frequently than A. fumigatus (Radwan et al., 2018b).
Yeasts can cause diseases in both humans and animals such as thrush, disseminated candidiasis, cryptococcosis, and mastitis (Radwan et al., 2014). In the past, C. albicans; the primary cause of candidiasis, was assumed to be the only pathogenic yeast of the genus Candida. However, it is now known that of the more than 200 species of Candida among them, seven are most frequently isolated and they have medical significance (Radwan et al., 2018b). Other candida species such as C. tropicalis, C. glabrata, C. parapsilosis, C. krusei and C. lusitaniae have also been implicated as causative agents of diseases (Radwan et al., 2014, 2016).
The increase of fungal resistance to classical drugs, the treatment costs, and the fact that most available antifungal drugs have only fungistatic effects justify the search for new strategies (Kotlarska et al., 2014). The essential oils (EOs) from many plants are known to possess antifungal activity. EOs are concentrated, hydrophobic liquid containing volatile aromatic and isolated phytochemicals compounds extracted from plants. They have been recognized for their potential antimicrobial role only in recent years, but the spectrum of activity and mechanisms of action remain unknown for most of them (Pinto et al., 2009).
Some EOs have shown important antifungal activity against yeasts, dermatophytes, and Aspergillus strains, which could predict therapeutic benefits, mainly for diseases with mucosal, cutaneous, and respiratory tract involvement (Bakkali et al., 2008). Previous studies have reported antifungal activity for eugenol (one of the main constituents of oregano, thyme, rosemary and clove EOs) against yeasts and filamentous fungi including food-borne fungal species (Radwan et al., 2018a). Clinical experience showed that the efficacy of antimicrobial agents depends not only on their direct effect on a given microorganism but also on the functional activity of the host immune system (Tullio et al., 2012).
The internal transcribed spacer (ITS) region is needed for accurate identification of fungal spp. as they amplified and sequenced the ITS region, ITS 1-5.8S-ITS 2 that isolated from clinical isolates of aspergilli and other fungi (El-Hamaky et al., 2016). The ITS regions are located between the 18S and 28S rRNA genes and offer distinct advantages over other molecular targets including increased sensitivity due to the existence of approximately 1000 copies per genome (Radwan et al., 2018c). The sequence variation of ITS regions has led to their use in phylogenetic studies of many different organisms (Mirhendi et al., 2007).
The present study was proposed to evaluate the antimycotic effect of some EOs (thyme, cinnamon and anise) on the molds and yeasts isolates associated with respiratory problems in broiler chickens. Additionally, the ability of the ITS gene PCR technique to identify selected molds and yeasts on the species as well as strain levels was investigated.
MATERIAL AND METHODS
Fungal isolates and their phenotypic identification
A total of 51 fungal isolates (36 molds and 15 C. albicans) were used in the current study. These isolates were previously isolated (Radwan et al., 2016) from broiler chickens suffered from respiratory disorders at different ages in Beni-Suef and El-Fayoum Governorates. The mold isolates included 10 A. flavus, 10 A. niger, and 16 A. fumigatus. All mold isolates were identified morphologically by examination of mycelial morphology and the reverse colour of colonies as well as microscopical examination of lactophenol cotton blue stained smears. Meanwhile, C. albicans isolates were identified by colonial morphology, microscopical examination of Gram`s stained smears, and biochemical tests including sugars fermentation test (glucose, lactose, maltose and galactose) and urease test according to Radwan et al. (2014) and Refai et al. (2014).
The antifungal activity of thyme, anise and cinnamon EOs
The antifungal activity of thyme, anise, and cinnamon EOs (Herbal Global Co., Egypt) against the fungal isolates was investigated by using the agar dilution method as previously described (Radwan et al., 2018a). Briefly, the tested fungi were grown on Sabouraud dextrose agar medium (SDA, Oxoid) at 35˚C for 48 hrs. Cells were suspended in normal saline (0.9% NaCl), and the suspension was adjusted to 1×106 CFU. SDA was prepared and autoclaved at 121˚C for 15 min. and kept at 55˚C. The tested EOs were sterilized by filtration (0.45 µm) and then mixed with SDA according to the tested concentrations (0.05, 0.1, 0.2, 0.3, and 0.5%). Then, 10 milliliters of the oil-agar medium were poured into sterile petri dishes and solidified. Equal amounts of the fungal suspensions were inoculated with multiple inoculators and speared onto the agar plates. The plates were then incubated at 35˚C for 24 hrs and then examined daily for 8 days.
Molecular characterization of selected fungal isolates
Internal transcribed spacer gene polymerase chain reaction (ITS-PCR)
PCR was applied on 8 fungal isolates included 6 Aspergillus spps. (2 A. flavus, 2 A. niger and 2 A. fumigatus) as well as 2 C. albicans. Genomic DNA was extracted using Gene-JET Genomic DNA Purification Mini Kit according to the manufacturer’s instructions. Extracted DNA was kept at -80°C until used in PCR amplification. Primers targeting the ITS gene were used to identify Aspergillus species and C. albicans isolates (forward: 5’-ATCCGCTATTTACCCAGTGG-3’ and reverse 5’- GCTGTAAACGAACTCGCCAC -3’) (Mirhendi et al., 2007). The reaction was performed in a volume of 50 µl consisting of 10 µl of 10X PCR master mix, 1µl of each 20 pmol primers, 5µl of DNA extract, and the volume was completed to 50µl using sterile deionized water. The thermal profile consisted of primary denaturation at 94˚C for 5 min., 35 cycles of amplification; denaturation at 94˚C for 30 sec., primer annealing at 56˚C for 45 sec., and extension at 72˚C for 1 min. followed by a final extension step at 72˚C for 7 min. PCR products were visualized using 1% agarose stained with ethidium bromide using a UV transilluminator.
Gene sequencing and sequence analysis
For gene sequencing, the target bands of the specific size of ITS gene regions were excised from the gel and purified with the QIAquick gel extraction kit (Qiagen, Valencia, CA) according to the manufacturer instructions and the DNA was quantified. A purified PCR product was sequenced in forward and reverse directions at Macrogen Inc. Korea. A BLAST® analysis was initially performed to establish sequence identity to GenBank accessions. Comparative phylogenetic analyses and determination of the genetic relationship of fungi and yeast were conducted. The evolutionary analyses were conducted in MEGA X and inferred using the Neighbor-Joining method. The analysis involved 41 nucleotide sequences with a total of 609 positions in the final dataset. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) (Kumar et al., 2018).
RESULTS
Morphotypic characterization of the fungal isolates
The fungal isolates were morphotypically characterized and the fungal species were confirmed. Colonies of A. niger isolates showed the characteristic black dotted surface as conidia were produced. Microscopically, A. niger exhibited septate hyphae, long conidiophores that supported large spherical vesicles that gave rise to large metulae and smaller phialides (Figure 1A and B). The A. fumigatus colonies were fluffy to granular, white to blue green with white periphery. Microscopical appearance showed septated hyphae, short conidiophores expanding into a large, dome-shaped vesicle having bottle-shaped phialides (Figure 1c and D). A. flavus growth was powdery yellow green colonies and microscopical globose or sub-globose vesicles producing phialides in two rows were observed (Figure 1E and F). Meanwhile, C. albicans were creamy smooth, glistening, and soft. Strongly gram positive budding yeast cells (blastoconidia) of 2-4µm diameter were seen (Figure 1G and H).
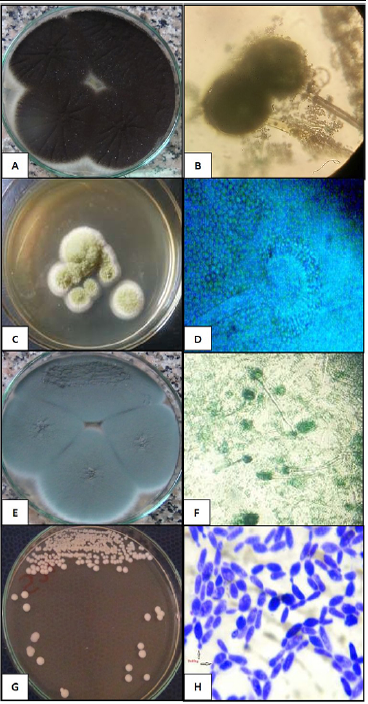
Figure 1: Colonial and microscopic characterization of the fungal and yeast isolates. (A and B) black dotted surface colonies of A. niger and septate hyphae, long conidiophores with large metulae and smaller phialides (40×). (C and D) A. flavus powdery yellow green colonies and microscopical globose vesicles producing phialides in two rows were observed (40×). (E and F) fluffy to granular white to blue green colonies of A. fumigatus colonies and septated hyphae, short conidiophores and bottle-shaped phialides (20×). The C. albicans creamy smooth glistening colonies and strong gram positive budding yeast cells (40×).
Antifungal effect of EOs against molds and yeast isolates
The antifungal activities of thyme, anise, and cinnamon oils at concentrations of 0.05, 0.1, 0.3, and 0.5% against the different fungal isolates were investigated using the agar dilution method. Thyme oil completely inhibited the growth of all the tested fungal isolates at concentrations of 0.2, 0.3 and 0.5% until 8 days after cultivation. Meanwhile, a concentration of 0.1% inhibited the growth of all A. niger and C. albicans isolates (100%) as well as 40% and 81.2% of A. flavus and A. fumigatus isolates, respectively. The growth of other non-affected isolates appeared on the 2nd and the 3rd days after cultivation. On the other hand, the concentration of 0.05% had no antifungal effect on all the tested isolates (Table 1).
Anise oil completely inhibited the growth of all the tested fungal isolates at a concentration of 0.5% only while concentrations of 0.3% inhibited the growth of 80%, 90%, 43.7%, and 66.7% of A. niger, A. flavus, A. fumigatus, and C. albicans, respectively. However, the growth of other non-affected isolates appeared at the 4th, 4th, 3rd, and 2nd days after cultivation, respectively. On the other hand, concentrations of 0.2, 0.1 and 0.05% had no antifungal effect on all the tested isolates (Table 1).
Cinnamon oil completely inhibited the growth of all the tested fungal isolates at concentrations of 0.1, 0.2, 0.3, and 0.5% while concentration of 0.05% inhibited the growth of 40, 20, 62.5 and 86.7% of A. niger, A. flavus, A. fumigatus, and C. albicans, respectively and the growth of other non-affected isolates appeared within the 1st and 2nd days after cultivation (Table 1).
ITS gene-based molecular identification of fungal isolates
PCR detection of the ITS gene
PCR was applied on 6 Aspergillus spps. (2 A. flavus, 2 A. niger and 2 A. fumigatus) as well as 2 C. albicans. The results revealed detection of 570-600 bp ITS gene fragment in all tested isolates.
Phylogenetic and sequence analysis of the fungal ITS gene
Generally, the phylogenetic analysis confirmed the phenotypic characterization, and each Aspergillus spp. or C. albicans strain clustered together with the relevant species (Figure 2). The sequence of the internal transcribed spacer 1 region (ITS-1) of A. niger isolates; KX059449-Egypt/BSU-4, and KX059450-Egypt/BSU-5 were found to be 99%, identical to previously identified A. niger strains KUASR15-MN187307, CPO MARH7A -MN944731, and strain CMXY5474-MG991588. The tested A. flavus isolates; KX059446. Egypt/BSU-1 and KX059447.Egypt/BSU-2 were found to be 100% identical to the A. flavus strains VCG1-CP051097.1, BB-1-MT584825.1, and strain L-3464/2012-MN533851.1. The sequence of the A. fumigatus isolate KX059448-Egypt/BSU-3 was 99% identical to reference A. fumigatus strains RS 20-MK267099 and MEFC011-MK732093. Finally, the isolated C. albicans ITS genes; KX059451-Egypt/BSU-6 and KX059452-Egypt/BSU-7, showed relatedness to the reference strains LEMI9268C-KF241846 and CBS:5722-KY101884 (Table 2).
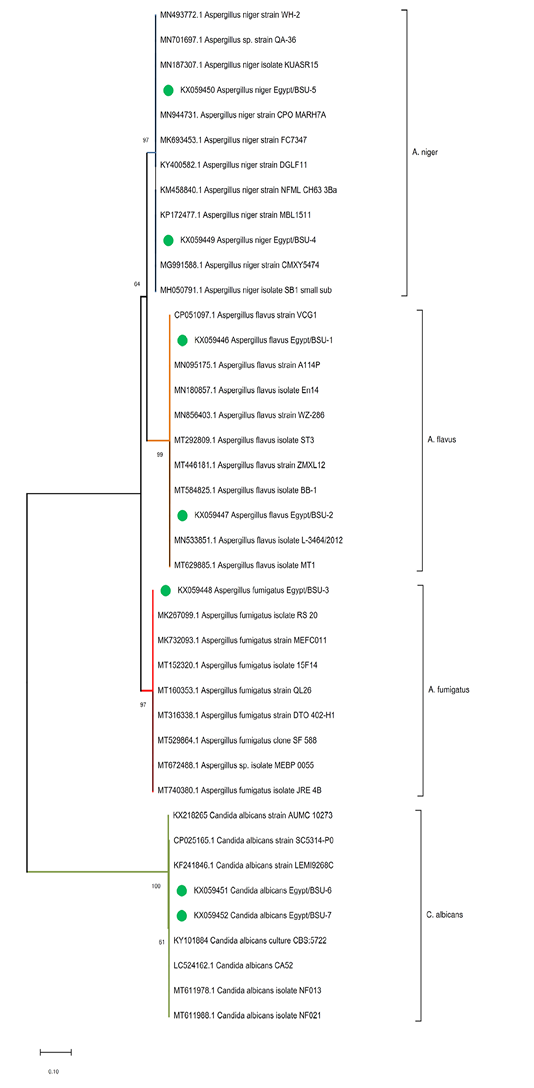
Figure 2: Phylogenetic tree of the ITS gene of the isolated fungi. The evolutionary analyses were conducted in MEGA X and inferred using the Neighbor-Joining method. The analysis involved 41 nucleotide sequences with a total of 609 positions in the final dataset. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches.
Table 1: Antimicrobial activity of different concentrations of thyme, anise, and cinnamon essential oils.
| Oil | Concentration |
A. niger (no. 10) |
A. flavus (no. 10) |
A. fumigatus (no. 10) |
C. albicans (no. 15) |
| Thyme | 0.05% |
+ (100%)(1:3 days)a |
+ (100%) (1:2 days) | + (100%) (1:2 days) | + (100%) (1:4 days) |
| 0.1% |
-b |
+ (60%) (2:4 days) | + (18.8%) (3:4 days) | - | |
| 0.2% | - | - | - | - | |
| 0.3% | - | - | - | - | |
| 0.5% | - | - | - | - | |
| Anise | 0.05% | + (100%) (1:3 days) | + (100%) (1:2 days) | ||
| 0.1% | + (100%) (1:4 days) | + (100%) (1:3 days) | |||
| 0.2% | + (100%) (1:4 days) | + (100%) (1:3 days) | |||
| 0.3% | + (20%) (4:5 days) | + (10%) (4:5 days) | + (56.3%) (3:5 days) | + (33.3%) (4:5 days) | |
| 0.5% | - | - | - | - | |
| Cinnamon | 0.05% | + (60%) (2:5 days) | + (80%) (1:4 days) | + (37.5%) (2:3 days) | + (13.3%) (1:4 days) |
| 0.1% | - | - | - | - | |
| 0.2% | - | - | - | - | |
| 0.3% | - | - | - | - | |
| 0.5% | - | - | - | - | |
aThe symbol “+” indicated fungal growth, followed by % of isolates showed growth, and the days at which growth appeared between brackets. bThe symbol “+-” indicated no fungal growth.
Table 2: Nucleotide identities of the molds and yeast isolates. The isolates characterized in this study are followed by red dots.
| Strain | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 14 | 14 | 15 | 16 | 17 |
|
KX059448 A. fumigatus Egypt/BSU-3● |
|||||||||||||||||
|
MK732093 A. fumigatus- MEFC011 |
100 | ||||||||||||||||
|
MK267099 A. fumigatus - RS 20 |
100 | 100 | |||||||||||||||
|
KX059447 A. flavus Egypt/BSU-2● |
86 | 86 | 86 | ||||||||||||||
|
MN533851 A. flavus - L-3464/2012 |
86 | 86 | 86 | 100 | |||||||||||||
|
MT584825 A. flavus isolate BB-1 |
86.1 | 86.1 | 86.1 | 100 | 100 | ||||||||||||
|
CP051097 A. flavus strain VCG1 |
86.1 | 86.1 | 86.1 | 100 | 100 | 100 | |||||||||||
|
KX059446 A. flavus Egypt/BSU-1● |
86.1 | 86.1 | 86.1 | 100 | 100 | 100 | 100 | ||||||||||
|
KX059450 A. niger Egypt/ BSU-5● |
90.3 | 90.3 | 90.3 | 88.2 | 88.2 | 88.2 | 88.2 | 88.2 | |||||||||
|
MN944731 A. niger- CPO MARH7A |
90.3 | 90.3 | 90.3 | 88.2 | 88.2 | 88.2 | 88.2 | 88.2 | 99.8 | ||||||||
|
MN187307 A. niger- KUASR15 |
90.3 | 90.3 | 90.3 | 88.2 | 88.2 | 88.2 | 88.2 | 88.2 | 100 | 99.8 | |||||||
|
MG991588 A. niger- CMXY5474 |
90.3 | 90.3 | 90.3 | 88.2 | 88.2 | 88.2 | 88.2 | 88.2 | 100 | 99.8 | 100 | ||||||
|
KX059449 A. niger Egypt/ BSU-4● |
90.3 | 90.3 | 90.3 | 88.2 | 88.2 | 88.2 | 88.2 | 88.2 | 99.8 | 99.8 | 99.8 | 99.8 | |||||
|
KX059451 C. albicans Egypt/ BSU-6● |
59.2 | 59.2 | 59.2 | 57.4 | 57.4 | 57.4 | 57.4 | 57.4 | 59.2 | 59.2 | 59.2 | 59.2 | 59.2 | ||||
|
KX059452 C. albicans Egypt/BSU-7● |
59.2 | 59.2 | 59.2 | 57.4 | 57.4 | 57.4 | 57.4 | 57.4 | 59.2 | 59.2 | 59.2 | 59.2 | 59.2 | 100 | |||
|
KF241846 C. albicans- LEMI9268C |
59.2 | 59.2 | 59.2 | 57.4 | 57.4 | 57.4 | 57.4 | 57.4 | 59.2 | 59.2 | 59.2 | 59.2 | 59.2 | 100 | 100 | ||
|
KY101884 C. albicans- CBS:5722 |
59.2 | 59.2 | 59.2 | 57.4 | 57.4 | 57.4 | 57.4 | 57.4 | 59.2 | 59.2 | 59.2 | 59.2 | 59.2 | 100 | 100 | 100 |
DISCUSSION
The essential oils have been investigated for their antimicrobial and antifungal activity as an excellent alternative for the chemical medication in recent years (Passone et al., 2012) due to their activity against yeasts, dermatophyte fungi, and Aspergillus spp. (Bakkali et al., 2008). In this study, the antifungal effect of 3 EOs (cinnamon, thyme, and anise) was investigated against A. niger, A. fumigatus, A. flavus, and C. albicans isolates from broiler chickens. Results revealed that thyme oil at concentrations as low as 0.2, and 0.3% completely inhibited the growth of different fungal isolates. The antifungal effect of thyme may be interpreted by the reduction of ergosterol content; the major sterol component in the fungal cell membrane. The distilled water of EOs inhibited the mycelial growth of A. flavus at various degrees, while the distilled water of thyme samples caused the highest inhibition percentage (100%) against A. flavus (Özkalp and Özcan, 2009).
Also, the susceptibility of fluconazole-resistant C. albicans, C. dubliniensis and other Candida spps. was previously reported where Eos of Zataria multiflora, Thymus kotschyanus, Cuminum cyminum, and Plargonium graveolens showed significant activity against C. albicans (Naeini et al., 2009). The antifungal effect of thymol against C. albicans was attributed to interfering with the envelope causing major morpho-structural deformities and envelope damage which was positively correlated with the thymol concentrations and time of incubation (Braga and Ricci, 2011; Pagiotti et al., 2011). Moreover, the thyme EO at sub-MIC/MIC concentrations was found to significantly enhancing the intracellular killing activity by polymorphonuclear granulocytes against C. albicans (Tullio et al., 2012).
Interestingly, it was observed a strong antifungal effect of cinnamon EO that completely inhibited the growth of different fungal isolates at a concentration as low as 0.1%. It was reported that cinnamon or cinnamon fortified with cinnamaldehyde has potent antifungal activity against a wide range of fungi including Penicillium spp., Aspergillus spp., and Candida spp., the concentrations were higher than the current study (4%, w/w) (López et al., 2005). Similarly, a 1% concentration of cinnamon, lavender, rosemary, and sage EOs completely inhibited the growth of spores of Aspergillus spp. and Penicillium spp. (Cvek et al., 2010)
However, the anise EO was able to completely inhibit the growth of different fungal isolates at a concentration of 0.5%, the lower concentrations had no or even mild effect on the fungal growth. Previous studies showed variable antimicrobial activity of the ethanolic and water extracts of anise seed using the disc diffusion method, where the ethanolic extract was more effective against all tested bacteria but not against C. albicans meanwhile the anise water extract failed to inhibit Gram-negative bacteria such as Pseudomonas aeruginosa and E. coli but it was effective against C. albicans (Gülçın et al., 2003). The minimal inhibitory concentration (MIC) and minimal fungicidal concentration (MFC) of anise oil were found to be 40 μl/ml against Penicillium spp. and A. niger while 60 μl/ml against P. chrysogenum (Matan and Matan, 2008).
The differences observed between findings of different studies has not been attributed to the total amount of the active chemical released but to the number of active compounds reaching the agar surface at a critical time (Gutierrez et al., 2010). Moreover, the differences in the purity of extracted oils and the total amount of the active ingredients as well as the method of testing (agar diffusion method, agar dilution method, or turbid metric monitoring of microorganism growth) may play a role in the required concentrations of cinnamon oil for complete inhibition of the fungal growth (Tullio et al., 2012).
The ability to distinguish between the various clinically relevant mold and yeast species may have diagnostic value. The ITS regions are located between the 18S and 28S rRNA genes and offer distinct advantages over other molecular targets including increased sensitivity due to the existence of approximately 1000 copies per genome (Radwan et al., 2018c). The sequence variation of ITS regions has led to their use in phylogenetic studies of many different organisms (Mirhendi et al., 2007).
In this study, the ITS region of the selected clinical isolates of Aspergillus spps. and C. albicans was amplified, sequenced, and compared with strain sequences in GenBank. All the selected isolates that were morphologically, microscopically, and biochemically identified as A. flavus, A. fumigatus, A. niger and C. albicans harbored ITS gene. The results confirmed that ITS gene PCR method is rapid and simple available tool for the identification of Aspergillus spp. especially the medically important species (Hinrikson et al., 2005).
On the nucleotide and amino acid levels, though minor variations within strains of the same species were observed (Khot et al., 2009), the ITS regions have been used as targets for phylogenetic analysis because they generally display sequence variation between species (Radwan et al., 2018c). Regarding other medically important fungi, one A. niger strain was found genetically related to the strain CMXY5474-MG991588 which were recovered from respiratory tract specimens in hospitals in China and Taiwan (Li et al., 2020). Similarly, the isolated A. fumigatus were related to medically important strain such as MEFC011-MK732093 that represents the recently reported emerging azole-resistant A. fumigatus (Colley et al., 2019). Also, C. albicans was related to clinical yeast isolates such as LEMI9268C-KF241846 from Brazil associated oral cavity, candidemia, and respiratory problems in human. These further indicate the probability of interspecies zoonosis of the A. niger and A. fumigatus in broiler chickens and humans (Radwan et al., 2018c). On the other hand, A. flavus isolates were genetically related to the strain VCG1-CP051097 associated with the production of highly toxic aflatoxins from maize crops (Fountain et al., 2014) indicating a probable feed source of the A. flavus in broiler chickens. In general, the ITS region sequences allowed the identification of Aspergillus spp. and C. albicans with the possible prediction of the potential source of the isolated molds and yeast.
Collectively the current study showed that cinnamon, thyme, and to some extent anise EOs have strong antifungal activity against molds and yeasts. Therefore, they may have the potential for use in the development of clinically useful antifungal preparations. In general, the ITS region sequences allow the identification of Aspergillus spp. and C. albicans with the possible prediction of the potential source of the isolated molds and yeasts.
ACKNOWLEDGMENTS
The authors would like to thank the staff at the Department of Bacteriology, Mycology, and Immunology, Faculty of Veterinary Medicine, Beni-Suef University, Egypt for their technical support.
AUTHOR’S CONTRIBUTION
Idea and Conceptualization: A.H.A. and I.A.R; Sample collection: M.M.A. and A.H.A.; Methodology and data analysis: A.H.A., I.A.R., M.M.A., and A.A. Original draft preparation: A.H.A and A.A. Reviewing and editing: A.H.A., I.A.R, M.M.A., and A.A.
CONFLICT OF INTEREST
The authors have declared no conflict of interest.
REFERENCES





