Advances in Animal and Veterinary Sciences
Research Article
Potentials of Glycoprotein Fraction in Successful Diagnosis of Sheep Haemonchosis
Nagwa Ibrahim Toaleb*, Eman H. Abdel-Rahman
Department of Parasitology and Animal Diseases, Veterinary Research Division, National Research Centre, 33 Bohouth St., Dokki, Giza, P.O. box 12622, Egypt.
Abstract | Haemonchus contortus (H. contortus) is a pathogenic nematode of small ruminants. In sheep, H. contortus causes anemia that decreases animal productivity and can ultimately lead to death. The objective of the current study was to evaluate the immunodiagnostic efficacy of four glycoprotein fractions for detection of anti-H. contortus IgG in sheep. Four fractions were isolated from crude extract of H. contortus adult worm by Con A affinity column chromatography. Characterization of the isolated four fractions; Glucose (Gluc), Galactose (gal), N-acetyglucosamine (GlucNAc), and Lactose (Lac) by SDS-PAGE showed three bands of 83, 66 and 24 kDa in Gluc fraction and three bands of the same molecular weights in GlucNAc fraction. Gal fraction resolved into four bands of 87, 70, 36 and 27 kDa, and Lac fraction resolved into two bands at 87 and 26 KDa, compared with many bands associated with crude antigen ranged from 100 to 24 KDa. The diagnostic potency of the isolated fractions was evaluated by indirect ELISA and Western blot. The highest diagnostic potentials are associated with Gluc fraction where its 24 kDa band reacted strongly with infected serum as proved by Western blot. Indirect ELISA based on Gluc fraction using positive and negative H. contortus sheep sera and sera infected with other parasites showed 100% sensitivity and 97.05% specificity. These percentages were confirmed by Western blot. The fraction recorded Positive Predictive Value (PPV) 92%, Negative Predictive Value (NPV) 100 % and diagnostic efficacy 97.8 %. Diagnosis of natural haemonchosis using Gluc fraction by indirect ELISA showed 56.83% infection percentage in examined sample. The present study proved the potency of Gluc fraction in detection of H. contortus specific IgG in natural sheep haemonchosis by indirect ELISA.
Keywords | Haemonchus contortus, Con A affinity chromatography, ELISA, Western blot, Glycoprotein fractions
Received | September 15, 2020; Accepted | December 03, 2020; Published | January 15, 2021
*Correspondence | Nagwa Ibrahim Toaleb, Department of Parasitology and Animal Diseases, Veterinary Research Division, National Research Centre, 33 Bohouth St., Dokki, Giza, P.O. box 12622, Egypt; Email: nagwaibrahim26@yahoo.com
Citation | Toaleb NI, Abdel-Rahman EH (2021). Potentials of glycoprotein fraction in successful diagnosis of sheep haemonchosis. Adv. Anim. Vet. Sci. 9(3): 335-343.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.3.335.343
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Lukkananukool et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Haemonchus contortus is a nematode parasite found in abomasum of sheep and goat, and feeds on blood (Edwards et al., 2016; Wang et al., 2017; Almeida et al., 2018). H. contortus infection causes huge economic losses, profitability reduction of the ruminant industry due to weight loss, acute anemia, edema, diarrhea, and lowering in milk and wool production (Emery et al., 2016; Gadahi et al., 2016a). This infection becomes a life threatening disease disturbing sheep population (Besier et al., 2016), because of massive mortality and morbidity rate in affected young animals (Dey et al., 2018). The mortality in lambs and kids in acute cases was ranging from 30% to 50% (Fawzi et al., 2014; Muchiuta et al., 2019). The worm anthelmintic resistanceis one of the factors that have supported this economic loss (Borges et al., 2020).
The ultrastructure of nematodes demonstrates different structures responsible for expression the different carbohydrate-containing glycoconjugates on their body, and their excretion/secretion products rich in glycans (Hassanain et al., 2009). These glycans have significant biological roles in the host–parasite relationship (Maizels and Hewitson, 2012; Ehsan et al., 2020). Parasite glycoproteins can direct and modify the development of immunity for evasion of host immune response and benefit the parasite (Harn et al., 2009). Through the glycan binding to host pattern recognition receptors causing a modified Th2-polarized immune response that support parasite survival in the host (van Die and Cummings, 2010; Wang et al., 2014; Sakthivel et al., 2018). Whereas, the secretory glycoprotein 55 kDa has ability to inhibit host neutrophils (Anbu and Joshi, 2008).
The specificity of carbohydrate to lectins demonstrated that D- mannose, α- d-glucose, and D-N-acetylglucosamin are the major carbohydrate epitopes on H. contortus proteins. Therefore, the major part of the carbohydrate moieties on these glycoproteins formed from N- linked oligosaccharides. These indicate that the host immune response against H. contortus is directed against the carbohydrate on the parasite (Schallig and van Leeuwen, 1996). Also, Van Stijn et al. (2010) reported that Gala1–3GalNAc epitopes are found on glycoproteins of H. contortus adult worms and ES antigens. Moreover, Trifucosylated glycans were found on the H. contortus H11 antigen (Roberts et al., 2013).
Recently, the attention of scientists has been focused on identification of immunogenic proteins to control of H. contortus infection and the possibility that parasite carbohydrate antigens can act as novel vaccine candidates (Paschinger and Wilson, 2015). Many studies in which many isolated glycoproteins H. contortus antigens were used for protection against H. contortus infection (Kalyanasundaram et al., 2015; Teixeiraet et al., 2019; Nobre et al., 2020). Based on the fact that vaccine candidates had diagnostic properties, the previous isolated glycoproteins could be successful used in diagnosis of sheep haemonchosis.
Early diagnosis of haemonchosis is important for effective treatment (Höglund et al., 2019; Naqvi et al., 2020a). The common method for diagnosis depends mainly on the fecal egg count and the clinical signs evaluation. These traditional methods are time consuming and lack the ability to diagnose the infection during the early stages before egg laying (Yang et al., 2017; Ljungström at al., 2018). Recently, different types of H. contortus antigens (larval, somatic and excretory-secretory products antigens) have been used for diagnosis of H. contortus infection by using immunoblotting and ELISA (Arab et al., 2013; Gowda, 2016; Kandil et al., 2017a, b). But these antigens shared in antigenic composition that leads to cross-reactivity in the diagnosis of infection (Roeber et al., 2013; Kandil et al., 2017b). To overcome this disadvantage a potential specific antigen based immunodiagnostic assay is need to improve control strategies (Wang et al., 2017). Cold Shock domain containing protein (CS) is one of HcESPs, act as immunodiagnostic antigen (Gadahi et al., 2016b). Also, recombinant cold shock H. contortus protein (rHc-CS) used as potential immunodiagnostic antigen to diagnosis H. contortus infection in goats at early and late infection (Naqvi et al., 2020a). Moreover, Naqvi et al. (2020b) used recombinant tropomyosin protein (Tropomyosin, one of excretory/secretory products) as diagnostic antigen for detection of H. contortus antibodies during early infection in goat by using western blotting and ELISA. Also, various recombinant parasite antigens used as potential diagnostic to detect H. contortus infection at early stage through these techniques (Aimulajiang, et al., 2020).
The current study aimed to present successful glycoprotein fraction for accurate diagnosis of sheep H. contortus infection by indirect ELISA.
MATERIALS AND METHODS
Ethical statement
H. contortus adult worms were recovered from slaughtered sheep in the government abattoir according to governmental regulations.
Parasite
Live H. contortus adult worms of both sexes were collected from abomasa of slaughtered sheep from local abattoirs in Egypt; El-Monieb, El-Bassatin and El-warrak. The collected worms washed with phosphate buffer saline (0.15 M, pH 7.3) to get rid of debris and stored at -20 °C (MAFF, 1986).
Blood samples
A total of 274 blood samples were collected from slaughtered sheep at different local abattoirs; El-Monieb, El-Bassatin and El-warrak in Egypt. The blood samples included; 183 random sheep blood samples. Whereas, the rest 91 blood samples including; 23 positive samples were collected from naturally infected sheep where H. contortus adult worms were collected from their abomasa used as true positive sheep sera (gold standard). 13 true negative samples from sheep with no adult worms of H. contortus in their abomasa and no other infection of parasites by postmortem examination and fecal analysis were used as uninfected control sheep (gold standard). 19 blood samples were collected from infected sheep with strongyloidiasis. In addition, 9 blood samples were collected from sheep infected with coccidiosis. Other, 9 blood samples from infected animals with fasciolosis as proved by their liver infected with Fasciola gigantica and fecal examination. Moreover, 18 blood samples were collected from sheep experimentally infected with Toxoplasma gondii; RH isolate from Department of Zoonosis, Veterinary Research Division, National Research Centre. All sera were separately stored at -20°C until use.
Preparation of H. contortus worm crude antigen
Antigen was prepared by homogenization of adult worms in phosphate buffer saline at 4˚C using a tissue-glass homogenizer, then sonication three times for 20 s each time at 100 m Amp by 150 ultra-sonication (Sanyo Gallenkamp PLC, UK), then centrifugation for 30 min at 12 000 rpm and 4 ˚C, then the supernatant was collected and kept at -20 ˚C (Prasad et al., 2008). The protein content of the collected supernatant (antigen) measured by Lowry method (Lowry et al., 1951).
Purification of glycoproteins by lectin affinity chromatography
The Concanavalin ensiformis (Con A) column obtained from Sigma Chem. Co. St. Louis was used for purification of glycoprotein antigens as previously described by Fukuda and Kobata (1993). The crude H. contortus antigen was applied to the column and the bound fraction was eluted with four types of sugars separately; glucose, N-acetylglucosamine, galacotse and lactose (Gluc- GlucNAc- Gal- and Lac). The protein content of fractions was checked by Lowry method (Lowry et al., 1951).
Characterization of isolated fractions
SDS-PAGE
Glycoprotein fractions, crude H. contortus antigen and molecular weight protein standards (Sigma, USA) were separately, electrophoresed on 10% gel of SDS- PAGE under reducing condition according to Laemmli (1970). After separation, the gel stained with silver stain according to Wray et al. (1981). The bands were analyzed and photographed by Molecular Imager Gel DocTM XR + with Image Lab Software (Bio-Rad).
Western blot
Immunoreactive bands in four isolated fractions and crude extract of H. contortus were identified by pooled positive sera of sheep naturally infected with haemonchosis in western blot. The four fractions, crude antigen and prestained protein Ladder (Vivantis Technologies) were blotted onto nitrocellulose membrane as described by Towbin et al. (1979) in a blotting system. The non-specific immunoreactive bands in the most potent diagnostic fractions were also identified in immunoblot using sheep serum samples infected with other parasitic infections as fasciolosis, toxoplasmosis, strongyloidiasis and coccidiosis, anti-sheep IgG horseradish peroxidase conjugate and 4-chloro-1-naphthol (Sigma) were used. Finally, the membrane was analyzed and photographed by Molecular Imager Gel DocTM XR + with Image Lab Software (Bio-Rad).
ELISA
Indirect-ELISA was used to evaluate the diagnostic potency of the four isolated fractions in detecting anti-H. contortus IgG in sheep sera naturally infected with haemonchosis. The fraction with highest diagnostic potency was used for diagnosis of haemonchosis in random sheep samples using indirect ELISA. The concentration of conjugate, antigen and tested sera were determined by checkerboard titrations. The optical densities (OD) were read at 450 nm with ELISA reader (BIO-TEK, INC., ELx, 800UV). The cut off value was calculated by mean OD values of negative control ± 3SD (Gowda, 2016).
Estimation of Sensitivity, specificity and predictive values of the most potent diagnostic fraction
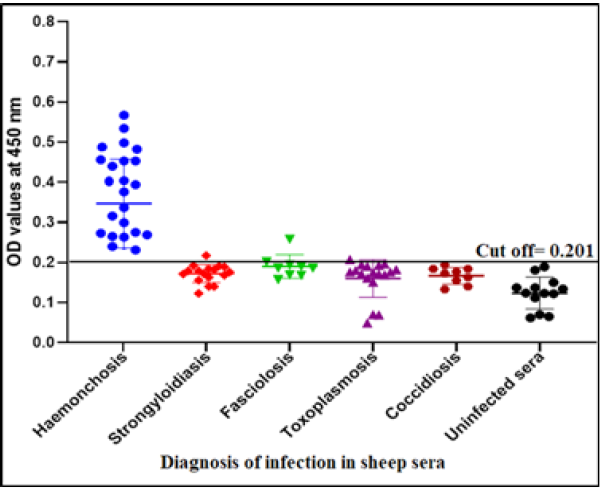
The cross reactivity with other parasitic infections than haemonchosis as strongyloidiasis (Strongyloides papillosus), fasciolosis (Fasciola gigantica), toxoplasmosis (T. gondii) and coccidiosis (Eimeria spp.) was estimated in the presence of positive sheep sera with haemonchosis (H. contortus) and negative sera using indirect ELISA. The sensitivity, specificity and predictive values were estimated as following formula (Parikh et al., 2008; Deo et al., 2019).
Sensitivity = Ntp/ (Ntp+Nfn)
Specificity = Ntn / (Ntn+Nfp)
Positive Predictive Value (PPV) = Ntp / (Ntp + Nfp)
Negative Predictive Value (NPV) = Ntn / (Nfn + Ntn)
Diagnostic Efficacy= (Ntn + Ntp) / (Ntp +Nfp+Ntn + Nfn)
Whereas, N (number of samples), tp (true positive), fp (false positive), tn (true negative) and fn (false negative).
Statistical analysis
The optical densities data were expressed as arithmetic mean ±standard deviation. The apparent prevalence parameter was analyzed using Chi square test by statistical computer package for social science (SPSS) version 15.
RESULTS
Lectin affinity chromatography profile of H. contortus adult worm antigen
Purification of H. contortus adult worms antigen by con A- affinity column chromatography resulted in four fractions; D- Glucose, D- Galactose, N-Acetylglucoseamine and Lactose fractions. The protein content of Gluc fraction was 78.3µg/ml, Gal-fraction was 57.9µg/ml, GlucNAc– fraction 62.5µg/ml and Lac fraction 60.3µg/ml, whereas, protein content of crude extract was 330.4 µg/ml.
Electrophoretic profile of H. contortus fractions
The electrophoresis profile of crude antigen showed 9 bands with a wide range of molecular weights, but the bands 100, 83, 70, 66, 56, 36, 31, 27 and 24 kDa seem to be the predominant bands as shown in Figure 1 Lane A. GlucNAc fraction separated into three bands with molecular weight 83, 66 and 24 kDa (Lane B). Gal fraction resolved into four bands of 87, 70, 36 and 27 kDa (Lane C). Gluc fraction displayed three bands with molecular weight 83, 66 and 24 kDa (Lane D). Lactose fraction was represented by two bands at molecular weight 87 and 26 kDa (Lane E) (Figure 1).
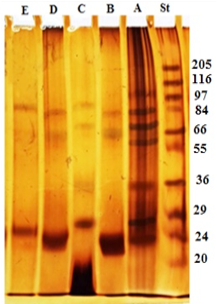
Figure 1: Electrophoretic profile of crude antigen of H. contortus adult worm (Lane A) and glycoproteins fractions; GlucNAc (Lane B), Gal (Lane C), Gluc (Lane D) and Lac (Lane E). Molecular weight protein standard (Lane St).
Western blot
The specific 24 kDa immunogenic reactive band in both Gluc and GlucNAc fractions reacted strongly with positive sheep sera naturally infected with H. contortus. Whereas, Gal and Lac fractions were very weakly reactive. The crude antigen was reactive with anti- H. contortus at four immunogenic bands 100, 83, 36, and 24 KDa (Figure 2).
Diagnostic potentials of H. contortus fractions
The diagnostic profile of the fractions showed the highest diagnostic potentials of Gluc fraction than other fractions using highly diluted antibodies 1: 65536 (Figure 3).
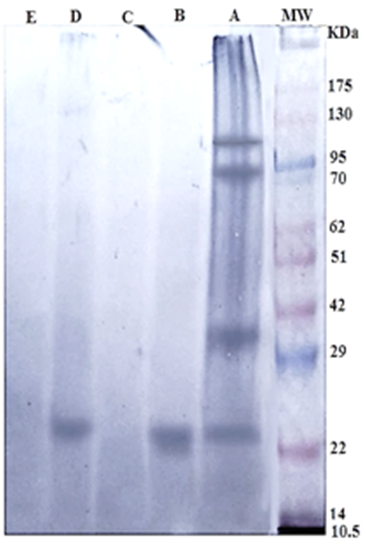
Figure 2: Immunogenic bands of H. contortus antigens identified by specific naturally infected sheep sera by Western Blot assay. Crude H. contortus antigen (Lane A), GlucNAc (Lane B), Gal (Lane C), Gluc (Lane D) and Lac (Lane E). Pre stained molecular weight Marker (Lane MW.).
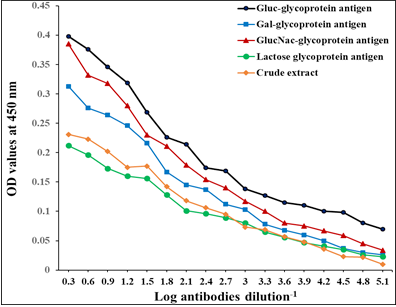
Figure 3: Diagnostic profile of four fractions compared to crude antigen in natural sheep haemonchosis. The difference is significant p ≤ 0.05.
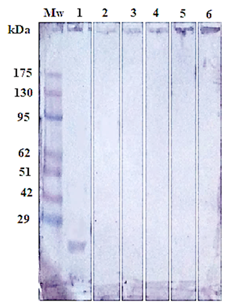
Figure 5: Immunoblotting of isolated Gluc fraction treated with; positive serum with haemonchosis (lane1), (strongyloidiasis (Lane 2), fasciolosis (Lane 3), toxoplasmosis (Lane 4), coccidiosis (Lane 5) and negative control (Lane 6). Pre stained molecular weight Marker (Lane Mw).
Specificity, sensitivity and predictive values of Gluc fraction
The success of Gluc fraction in detection of specific haemonchus IgG in sheep was assessed by indirect ELISA using different antibodies with other parasitic infections. The fraction recorded 97.05% specificity (Figure 4). Whereas, the fraction recorded 5.8% cross reactivity (false positive) with sheep serum infected with strongyloidiasis (n=19, one false positive for haemonchosis) and 11% with fasciolosis IgG (n=9, one false positive for haemonchosis). No cross reactivity was recorded with uninfected sheep sera (negative sera for H. contortus), and no cross reactivity with antibodies of toxoplasmosis and coccidiosis, (Figure 4). Serum samples of sheep infected with H. contortus, as proved by post mortem examination, reacted positively with Gluc fraction recording 100% sensitivity (Figure 4). The fraction recorded Positive Predictive Value (PPV) 92%, Negative Predictive Value (NPV) 100 % and diagnostic efficacy 97.8 %. The current results of sensitivity and specificity were confirmed by western blot (Figure 5).
Sheep haemonchosis infection percentage by indirect ELISA using Gluc fraction
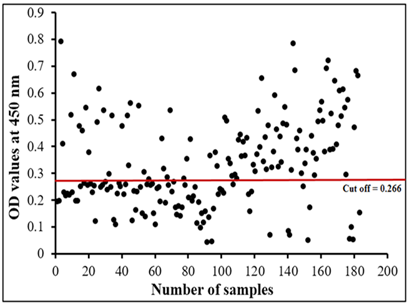
The assay recorded 56.83% infection percentage in the examined sample. OD values of the examined serum samples ranged from 0.045 to 0.795 (Figure 6).
DISCUSSION
In the current study, four glycoprotein fractions were isolated from whole worm crude extract. Electrophoretic profile of the four fractions showed that both Gluc and GlucNAc fractions have common three bands with molecular weight 83, 66 and 24 KDa. While Gal fraction resolved into four bands 87, 70, 36 and 27 KDa, and Lac fraction was represented by two bands at molecular weight 87 KDa and 26 KDa. Whereas, crude extract antigen separated into many bands at molecular weight range from 100 kDa to 24 KDa. These bands of crude extract were found in the range (14 to 216 kDa) previously described by Derbala and Abd El-Rahman (2001). Whereas, Anbu and Joshi (2008) isolated 55 kDa secretory glycoprotein (gp55) from E/S products of adult H. contortus by using Con a Sepharose column. While, Ashman et al. (1995) purified 70-90 kDa larval surface glycoprotein of H. contortus by size exclusion chromatography. Moreover, the galectin 11 and 14 ligands were isolated from H. contortus by the galectin affinity column (Sakthive et al., 2018). Whereas, Naqvi et al. (2020b) observed that rHc-Cs which purified as Histidine-tagged fusion protein, resolved into single band at 38 kDa, while native CS protein resolved at 20 kDa on 12% SDS-PAGE. The difference in molecular weight of glycoproteins bands may be due to the difference in origin of crude extract from which glycoproteins isolated, or antigen preparation, eluting buffers and type of column used in purification process.
In the current study, 24 kDa is immunoreactive band of that found in both Gluc and Gluc-NAc fractions which showed highest diagnostic potentials. This common band is mainly responsible for the diagnostic activities in both fractions. The difference in diagnostic properties of Gluc and Gluc-NAc fractions may be attributed to quantities of this band in both fractions. The band 24 kDa was previously isolated from H. contortus excretory-secretory products of adult worm (HcES-24) (Gadahi et al., 2016). And diagnostic 26-kD band was isolated from adult H. contortus by anion exchange chromatography and proved potency in early and late experimental sheep haemonchosis (Gomez-Mun˜oz et al., 2000; Arab et al. (2013) proved the potency of 26 KD which isolated from adult H. contortus by gel filtration chromatography using sephadex G 100 in the diagnosis of sheep haemonchosis.
In the present study Gluc fraction has been successfully detected specific antibodies of H. contortus with specificity 97.05% and sensitivity 100% using indirect ELISA. The results of specificity were confirmed by western blot. Antigen specificity is essential in accurate sero-diagnosis of diseases. This fact can be applied with all pathogens particularly parasites. Where cross reaction is a common trait among helminthes (Abdel-Rahman and Abdel-Meeged, 2000; Hassan et al., 2012; Shaapan et al., 2015) and consequently, isolation of specific antigen is a challenge.
In previous study, Naqvi et al. (2019) showed that no cross reactivity with the purified rHc-HCA59 antigen from the excretory and secretory products against infected sera with T. spiralis, F. hepatica and T. gondii. Moreover, our results agree with Naqvi et al. (2020b) who reported that western blotting results showed that rHc-TpMy was detected all positive sera of haemonchosis while no antibody detection was observed against negative sera. In addition, Naqvi et al. (2019) reported that combined use of western blot and indirect ELISA analysis are potential diagnostic tool for diagnosis of H. contortus infection in goat. In contrast to this, Kandil et al. (2017a) showed that there were cross reactivity with other cestodes, Moniezia expansa and F. hepatica against glutathione-S-transferase (GST) and recombinant protein (rhcp 26/23) antigens based on both indirect ELISA and immunoblot assays. Moreover, partially purified crude H. contortus antigen by gel filtration using Sephadex G-100, showed cross reactivity with other cestodes and F. hepatica in both indirect ELISA and immunoblot (Kandil et al., 2015). In addition, Kandil et al. (2017a) demonstrated that the recombinant H. contortus glutathione-S-transferase was showed high false positive with negative sheep sera and scored highest prevalence (90.8%) and sensitivity (90%) by indirect ELISA. Therefore, Waritani et al. (2017) explained that False positive/negative results are unwanted and may affect the efficacy of assays so, they can performed the optimization of antigen/antibodies concentration to improve the diagnostic potential of assay .
In current study Gluc-glycoprotein fraction record sensitivity 100% and specificity 97.05% which is higher than somatic antigen which recorded specificity 67.18% by indirect ELISA in previous study (Gowda, 2016). And it may be similar to rHc-TpMy-which showed 90% sensitivity and 100% specificity (Naqvi et al., 2020b). While, Song et al. (2018) consider that the sensitivity and specificity of assay affected by the type of antigens used. Whereas, Gomez et al. (1995, 2000) and Arab et al. (2013) suggested that the 26 kDa antigen of adult H. contortus has been seem specific for the diagnosis of H. contortus infection. On the other hand, Mir et al. (2008) showed that adult crude antigen scrod 72.22 % and 76.81 % sensitivity and specificity respectively by indirect ELISA. While, Sultan et al. (2012) reported that the sensitivity of crude adult worm antigen was 87.5% and specificity 75 %. Moreover, previous study Schallig et al. (1995) showed that crude somatic antigens based on ELISA recoded sensitivity 89.2 % and specificity 82.7 % for diagnosis haemonchosis in sheep.
The infection percentage of haemonchosis in the current study is 56.83%, Positive Predictive Value 92%, Negative Predictive Value was 100 % and 97.8 % diagnostic efficacy using indirect ELISA. These results indicate that this test indirect ELISA based on new Gluc-glycoprotein fraction is doing as good as “gold standard”. And other fractions recorded in previous studies, different infection percentages were recorded based on the used antigens and assays. 72.22 % sero-prevalence was observed with crude H. contortus somatic antigen (Mir et al., 2008). 58.66 % serum samples screened using somatic antigen (Gowda, 2016). Whereas, Hassan et al. (2019) reported that the sero-prevalence of haemonchosis among sheep was higher than goats and represented 64.48% and 41.91% respectively. Additional factor that is responsible for different infection percentages is body condition where, Brik et al. (2019) reported maximum prevalence 80.83% in sheep with poor body, (34.26%) in moderate body and 6.88% in sheep with good body. The difference could be also attributed to examine sample size.
CONCLUSION
Gluc fraction isolated from H. contortus adult worm antigen by Con A lectin column chromatography with immunoreactive band 24kDa was successfully utilized in the diagnosis of sheep haemonchosis with 100% sensitivity, 97.05% specificity, Positive Predictive Value 92%, and Negative Predictive Value 100 % with 97.8 % diagnostic efficacy using indirect ELISA. The immunogenic band of 24 kDa is mainly responsible for its potency. Further purification and characterization processes are needed to increase the specificity of the fraction and its diagnostic potency.
ACKNOWLEDGEMENTS
I thank National research Centre for provision of laboratory facilities.
AUTHOR’S CONTRIBUTIONS
The authors participated in planning and study design. TNI collected H. contortus adult worms from abomasa of slaughtered sheep and blood samples. TNI prepared crude antigen, performed lectins chromatography, SDS-PAGE and western blot assays. TNI and A-REH shared in ELISA. TNI and A-REH shared in analyzed data and writing the manuscript.
CONFLICT OF INTEREST
The authors have declared no conflict of interest.
REFERENCES