Advances in Animal and Veterinary Sciences
Research Article
Advances in Animal and Veterinary Sciences. 2 (1S): 13 – 16Special Issue – 1 (2014): (Infectious Diseases of Animals and Global Health
A Simple Innovative Modification to Enhance the Efficacy of the Complement Fixation Test
Shubhada Chothe, Hari Mohan Saxena*, Mudit Chandra
- Department of Veterinary Microbiology, College of Veterinary Science, Guru Angad Dev Veterinary and Animal Sciences University, Ludhiana
*Corresponding author: hmsaxena@yahoo.com
ARTICLE CITATION:
Chothe S, Saxena HM and Chandra M (2014). A simple innovative modification to enhance the efficacy of the complement fixation test. Adv. Anim. Vet. Sci. 2 (1S): 13 – 16
Received: 2013–11–07, Revised: 2013–12–22, Accepted: 2013–12–23
The electronic version of this article is the complete one and can be found online at
(
http://dx.doi.org/10.14737/journal.aavs/2014/2.1s.13.16
)
which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited
ABSTRACT
The Complement Fixation Test (CFT) is a serodiagnostic test of high sensitivity commonly used in the diagnosis of infectious diseases like Brucellosis. However, the conventional CFT (cCFT) may sometimes give false negative or false positive result and may lead to incorrect diagnosis. If the results are not read within 30 minutes of finishing the test, it tends to show the signs of negativity making it difficult to find the true results. In the present study, serum samples from Brucellosis affected or healthy cattle were subjected to a modified CFT (mCFT) where, in addition to the regular procedure of the CFT, anti–hemolysin antiglobulin {i.e., [F(ab’)2] of antibody against rabbit IgG}, was added to facilitate the reading of the test. The innovative modification showed a clear hemolysis in negative samples as the antiglobulin binds to the haemolysin leading to pairing of the antibody molecules, enhancing the complement mediated haemolysis. The novel method could help in differentiating the positive and negative samples more explicitly and the test could be read more accurately. In the modified CFT, the results could be recorded easily even after 2 hours of finishing the test, minimizing the chances of wrong diagnosis. The modified CFT with a better readout would enable more accurate serodiagnosis of infectious diseases.
INTRODUCTION
Complement Fixation Test (CFT) is often recommended as a confirmatory serological test for Brucellosis. The complement fixation test is widely used as the confirmatory test for confirming a diagnosis of brucellosis. Since only IgG1 isotype of antibody fixes complement well, the test specificity is high (Poester et al, 2010).
Stemshorn and Forbes (1985) demonstrated a sensitivity of 79% for CFT while carrying out a comparative study of standard serological tests for the diagnosis of bovine brucellosis. Gupta et al (2010) reported a sensitivity of 80 % for CFT while carrying out a comparative evaluation of recombinant BP26 protein for serological diagnosis of B. melitensis infection in goats.
The problems with CFT include the subjectivity of the interpretation of results, occasional direct activation of complement by serum (anticomplementary activity) and the inability of the test for use with haemolysed serum samples. However, in spite of the shortcomings, the complement fixation test has been considered as a confirmatory test in control / eradication programs (Poester et al, 2010). In the conventional CFT, there are chances of some degree of hemolysis occurring in positive samples too. If the results are not read within 30 minutes of finishing the test, it tends to show the signs of negativity making it difficult to find the true results. In the present study, a novel modification to the CFT – viz. addition of antiglobulin to hemolysin, was tested and the results were compared with those of the conventional CFT.
MATERIALS AND METHODS
Collection of Blood Samples for Serum
Blood samples from 200 Brucellosis suspected or healthy cattle were collected from the veterinary clinics, dairy farms and gaushalas, in and around Ludhiana, Punjab. All the animals were of age more than two years. About 10 ml of blood was collected aseptically from the jugular vein of the animal. Serum was collected by centrifuging the clotted blood at 3000 rpm for 15min.
Complement Fixation Test
The standard method of CFT as per the OIE Manual (OIE, 2009) was followed. Barbital (Veronal) Buffered Saline was used as the standard diluent for the CFT. Following was the composition of Veronal Buffer (VB; pH 7.4; Sodium 5, 5, Diethyl Barbiturate: 3.75g; 5, 5, Diethyl–Barbituric Acid: 5.75g; Magnesium Chloride: 1.68g; Calcium Chloride: 0.28g; Sodium Chloride: 85g). All the contents were dissolved in 500 ml of hot distilled water (80–90°C) for 10–15 mins. The final volume was adjusted to 3000 ml. This stock buffer was stored in a refrigerator. For making the working dilution of the buffer, one part of the stock buffer was mixed with four parts of cold distilled water and the final pH was adjusted to 7.4.
Blood from a healthy sheep, negative for anti–Brucella antibodies, was collected under aseptic conditions into equal volume of Alsever’s solution. It was mixed thoroughly and centrifuged at 12000 rpm for 10 mins. Thereafter, the supernatant was discarded along with the thin layer of white cells and the erythrocytes were washed twice, first with the PBS and then with the Veronal buffer by centrifugation at 5000 rpm for 10 mins. To make 1% suspension of sheep erythrocytes, 100 µl of erythrocytes were suspended in 9.9 ml of Veronal buffer (VB). The Alsever’s solution was autoclaved at 110°C and stored at 4°C. Following was the composition of the Alsever’s solution: Sodium Chloride: 4.2g; Dextrose: 20.5g; Citric Acid: 0.55g; Sodium Citrate: 8.09g; DW: upto 1000ml.
Haemolysin (anti–sheep erythrocyte antibody raised in a rabbit) was prepared by intravenous (i/v) inoculation of 1% sheep RBC’s in a healthy rabbit. The immunization for haemolysin was done for duration of 20 days as per the method described by Darter (1953). The rabbit was bled by cardiac puncture and serum was separated. High titre serum was stored at 4°C in a refrigerator.
Serum from a guinea pig used as the source of good quality complement, was obtained from Indian Veterinary Research Institute, Izatnagar. The guinea pig serum was stored at 4°C in a refrigerator.
Haemolysin Titration
To obtain a dilution of haemolysin for use in the test proper, a serial dilution of haemolysin was prepared in VB ranging from 1:10 to 1:5120. An equal volume of 1% sheep erythrocyte suspension was added to each dilution and incubated for 30 min at room temperature. An erythrocyte control was set up. The highest dilution of haemolysin that produced 100% haemolysis was taken as one unit of the haemolysin. Two units of haemolysin were used in the test proper.
Complement Titration
For the titration of the complement, a dilution of haemolysin containing 2 haemolytic units was prepared. The haemolysin was inactivated by heating at 56°C for 30 min. Serial dilutions of complement were made in VB ranging from 1:10 to 1:5120 in a ‘U’ bottom 96 well plate. Equal amounts of haemolysin and 1% sheep erythrocyte suspension were added to each dilution of the complement. The plate was then incubated for 2 hours at room temperature. The highest dilution giving complete haemolysis was considered as the titre of the complement. In the test proper, two units of complement were used.
Antigen Titration
A known positive serum was used for titration of antigen. The serum was inactivated at 56° for 30 min and diluted 1:2 in VB. Further, 2 fold serial dilutions of the inactivated serum were made in VB and 25µl was dispensed in the vertical rows of the plate. Two – fold serial dilutions of the antigen were made and dispensed in the horizontal rows of the plate. About 25 µl of the complement having a final dilution of 1:320 was added to each well. An anti–complementary control for each antigen dilution containing VB, antigen and complement was incubated at 37°C. About 25 µl of the sensitized sheep erythrocytes was added to each well and incubated at 37°C for 30 min. The highest dilution of the antigen giving complete haemolysis was selected as the dilution for antigen in the test proper.
Procedure of CFT
OIE method (OIE, 2009) was followed. Test serum samples were inactivated at 56°C for 30 min. Each serum sample was serially diluted in VB in horizontal rows in a ‘U’ bottom–96 well microtitre plate. To prepare the serial dilutions of the test serum, 25 µl of VB was added to each well in the horizontal row except in the first well in which 50 µl of VB was added. 5µl of the inactivated test serum was added to the first well and serial dilution was carried out by transferring 25 µl of the content to the next well and discarding 25 µl from the last well. Equal volume (25 µl) of Brucella abortus plain antigen was added in each well, followed by 25 µl of complement in all the wells. The plate was incubated at 37°C for 60 min to allow fixation. Freshly prepared 50 µl sensitized RBCs were added to each well and further incubated at 37°C for 30 min. The plates were checked for the presence of hemolysis. The absence of anti–complementary activity was checked in the controls.
Modified CFT
Serum samples from six Brucellosis positive and six negative cattle, aged more than 3 years, were collected. The samples were declared as positive or negative based on the results of 3 serological tests – Rose Bengal Plate Test (RBPT), Standard Tube Agglutination Test (STAT) and Enzyme Linked Immunosorbant Assay (ELISA). These sera were incubated at 56°C for 30 minutes to inactivate the complement. Sera from all the 12 cattle were subjected to the modified CFT. F(ab’)2 fragment of affinity purified antibody against rabbit IgG (H+L) was added at a dilution of 1:100 just before the last step of incubation in the end.
The test was carried out as per the procedure recommended by the OIE (2009), except the novel modification of adding affinity purified antibody to rabbit IgG (H+L), F(ab’)2 along with the sensitized RBC’s followed by incubation for 30 minutes at 37°C. The test was applied to the serum samples in duplicates for the serum dilutions ranging from 1:10 to1:20480 for each sample. The controls were set up as per the standard method. One set of positive and negative control was set up for the entire procedure without adding anti rabbit IgG.
Test Protocol
The test was carried out in a 96 well micro titre plate. 25 µl of Veronal buffer (VB) was added to all the wells expect the first well of the horizontal row in which 50 µl of VB was added. 5µl of the test serum was added in first well of each row and serial dilutions were made in all horizontal rows, starting from first well and finishing with last one, from which 25 µl was removed. 25 µl of antigen (neat) was added to each well. The plate was then incubated at 37°C for 3–4 hours. Dilution of complement was made in VB to contain 2 units and it was added to all the wells. The contents were mixed by gently shaking the plate. It was then incubated at 37° for 2 hours. 50 µl of sensitized RBC’s were added to all the wells, followed by 25 µl of 1:100 diluted anti rabbit IgG (Fab)2. The contents were mixed by gentle shake and the plate was incubated at 37° for 30 minutes. Duplicate readings for each sample were taken. Results were read after 30 minutes of incubation. Known positive and known negative samples were set as control, on which both conventional CFT and modified CFT were performed to aid in visualizing the difference between the two tests.
Setup of Samples for the Modified CFT
Serum samples from 12 cattle were studied, out of which 6 were positive and 6 were negative for Brucellosis by RBPT, STAT and ELISA. Rows were named as A, B, C up to X for identification. Positive and Negative controls and complement control were set separately in a 96 well microtitre plate.
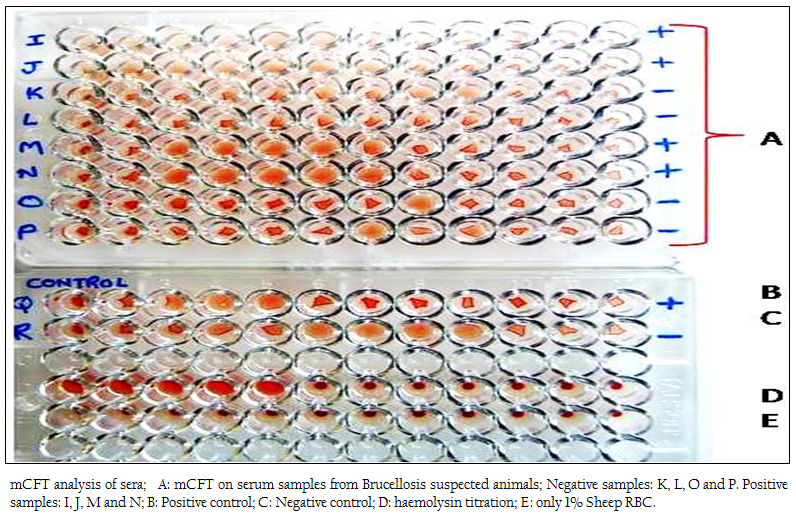
Figure 1: mCFT analysis of sera; A: mCFT on serum samples from Brucellosis suspected animals; Negative samples: K, L, O and P. Positive samples: I, J, M and N; B: Positive control; C: Negative control; D: haemolysin titration; E: only 1% Sheep RBC.
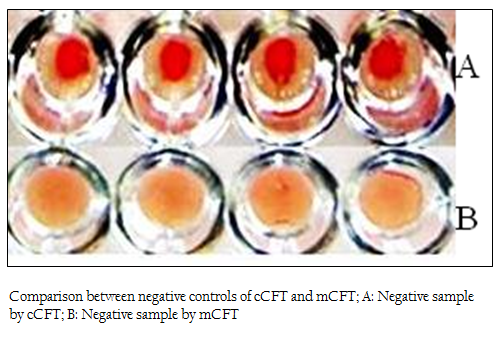
Figure 2: Comparison between negative controls of cCFT and mCFT; A: Negative sample by cCFT; B: Negative sample by mCFT
RESULTS AND DISCUSSION
The positive and negative results of the samples by modified CFT were compared with the results of conventional CFT (Figures 1 & 2). It was observed that this modification could differentiate the positive and negative samples more explicitly and the test could be read more accurately. In the conventional technique, atypical reactions in the CFT sometimes causes difficulties in diagnosis (Chappel et al., 1978) and may give false negative appearance to the sample (Radiostits et al., 2000). In the conventional test, there are chances of some degree of hemolysis occurring in positive samples too. The modification showed a clear hemolysis in negative samples as the anti–Rabbit antibody binds to the haemolysin, enhancing the haemolysis procedure. Also it was observed that, the conventional test results if not read within 30 minutes of finishing the test, tend to show signs of negativity making it difficult to determine the true results. This limitation was not observed in m–CFT, and the results could be recorded even after 2 hours of finishing the test.
The complement fixation test is widely used as the confirmatory test for brucellosis. Since only IgG1 isotype of antibody fixes the complement well, the test specificity is high (Poester et al., 2010). Although the complement fixation test has been and is a valuable asset in control/eradication programs, the problems with the test include the subjectivity of the interpretation of results (Poester et al., 2010). The modification described in this study can enhance the accuracy of the test and can be valuable in the control of infectious diseases like brucellosis by minimizing the false negative results encountered with the conventional CFT
REFERENCES
Chappel RJ, McNaught DJ, Bourke JA and Allan GS (1978). Comparison of the results of some serological tests for bovine brucellosis. J. Hyg. (Lond) 80(3): 365 – 371.
http://dx.doi.org/10.1017/S0022172400024827
http://dx.doi.org/10.1017/S0022172400024815
Darter LA (1953). Procedure for production of anti–sheep hemolysin. J. Lab. Clin. Med. 41(4): 653 – 654.
PMid:13045019
Gupta VK, Kumari R, Vohra J, Singh SV and Vihan VS (2010). Comparative evaluation of recombinant BP26 protein for serological diagnosis of Brucella melitensis Infection in goats. Small Rum Res. 93: 119 – 125.
http://dx.doi.org/10.1016/j.smallrumres.2010.05.009
OIE (2009). Bovine Brucellosis. In: Manual of the Diagnostic Tests and Vaccines for Terrestrial Animals, Office International Des Epizooties, Paris, France, volume 1: 624 –659.
PMid:20049821
Poester FP, Nielsen K, Samartino LE and Yu WL (2010). Diagnosis of Brucellosis. The Open Vet. Sci. J. 4: 46 – 60.
Radostits OM, Gay CC, Blood DC, and Hinchcliff KW (2000). Veterinary Medicine, A textbook of the Diseases of Cattle, Sheep, Pigs, Goats and Horses. Harcourt Publishers Limited, London, 867 – 882.
Stemshorn, BW, Forbes LB, Eaglesome MD, Nielsen KH, Robertson FJ and Samagh BS (1985). A comparison of standard serological tests for the diagnosis of bovine brucellosis in Canada. Can J Comp Med. 49: 391 – 394.
PMid:4075239 PMCid:PMC1236197