Advances in Animal and Veterinary Sciences
Case Report
Lethal Adverse Consequence of an Anticoccidial Therapy with Sulfa Drugs in Inland Bearded Dragon (Pogona vitticeps)
Lai O.R. 1, Tinelli A.1*, Gelli D. 2, Escudero E. 3, Crescenzo G. 1
1Department of Veterinary Medicine, University of Bari “Aldo Moro”, s.p. Casamassima km 3, Valenzano 70010, Bari, Italy; 2Department of Animal Medicine, Productions and Health, University of Padua, Legnaro 35020, Padua, Italy; 3Department of Pharmacology, Faculty of Veterinary Medicine, University of Murcia, 30100 Murcia, Spain.
Abstract | Coccidia have been recognized as a salient disease-causing parasite of captive reptile species, and this is linked with high mortality rate in youthful subjects. In this paper authors report the mortal effect of an anticoccidial therapy with sulfamides in a youthful bearded dragon (Pogona vitticeps) prescribed by a reptiles’ non-experienced practitioner. A formulation containing diaveridine 0.5% and sulfadimetoxine 0.5% was given orally at the dosage of 71.4 mg/kg for both drugs, q 24 h for 7 days. No particular advisement was given to the owner about the exigency to integrate fluids assumption during therapy to avoid possible injurious effect of sulfa drugs on urinary system. During therapy, the animal became malnourished and lethargic, so it was referred to authors. Despite the therapy instituted on the histopathological examination of renal samples showed large multifocal areas of coagulative necrosis, and tubular lumina plugged with cell casts, crystals, calcium salts, erythrocytes leukocytes, and amorphous precipitates. Liver samples showed diffuse fatty change and necrosis of hepatocytes, and lungs presented hyperaemic areas. The final recommendation for practitioners interested in exotic and reptile medicine practice is therefore to not device therapeutic protocols, but rather to refer to specially designed literature or more competent colleagues.
Keywords | Sulfonamides; Pogona vitticeps; Coccidia; Histopathology.
Received | October 08, 2020; Accepted | October 19, 2020; Published | December 05, 2020
*Correspondence | Antonella Tinelli, Department of Veterinary Medicine, University of Bari “Aldo Moro”, s.p. Casamassima km 3, Valenzano 70010, Bari, Italy; Email: antonella.tinelli@uniba.it
Citation | Lai OR, Tinelli A, Gelli D, Escudero E, Crescenzo G (2021). Lethal adverse consequence of an anticoccidial therapy with sulfa drugs in inland bearded dragon (pogona vitticeps). Adv. Anim. Vet. Sci. 9(1): 21-25.
DOI | http://dx.doi.org/10.17582/journal.aavs/2021/9.1.21.25
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2021 Tinellii et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
The pet reptile trade is a multimillionaire business, and the popularity of reptiles as pets has resulted in a need for veterinarians with preparation in their medical management. It involves knowledge of husbandry and medical needs of approximately 7,500 vertebrate species, highly diversified, having biological and medical peculiarities that differ both between and within major groups. Therefore, reptiles medicine requests veterinarians experienced in caring for these exotic species to continually gather knowledge concerning both their proper husbandry and medical and/or surgical casualties (de la Navarre, 2006; Jacobson et al., 2006).
The inland bearded dragon (Pogona vitticeps) is a diurnal, oviparous, omnivorous, agamid lizard native to inland Australia. Due to its placid nature, ease of adaptation to captive conditions and relative hardiness, it has rapidly become popular in captive collections and in pet business (Eliman, 1997). The admittance of this species in captivity has seen the concomitant need for control of the species’ parasites. The commonest endoparasites detected in bearded dragons are coccidia. Only two coccidia parasitizing P. vitticeps have been described, Isospora amphiboluri and Eimeria pogonae. I. amphiboluri is a parasite with strict host specificity for the Australian agamid lizard from the genus Pogona, reported in wild and captive populations (Walden, 2009). In the life cycle of the endogenous stage, the parasite appears to be confined to the intestinal epithelial cells. On the other hand, Eimeria pogonae has recently been reclassified into the correct genus Choleoeimeria, which is restricted located to the gallbladder epithelium of reptiles, mainly lizards; there it causes epithelial anomalies in gallbladders, with chronic inflammation induced by group of oocysts accompanied with precipitates of tissue debris and gallstones (Szczepaniak et al., 2009). Oocysts of both species can be observed in fecal samples.
Isosporosis of young bearded dragons is commonly associated with high mortalities (>15%), while in adult individuals I. amphiboluri seem to be a low pathogenic parasite, even if it cause reduced fecundity and declining health in older dragons (Kim et al., 2002; Walden & Mitchell, 2012; Jańczak et al., 2014). In a study it was found that more than 23% of adult bearded dragons in breeding colonies shed this organism, and this is presumably underestimated because it was based on a single faecal specimen examination (Walden, 2009). I. amphiboluri has been known for over 40 years, however no study has been conducted to assess the effectiveness of the various therapeutic protocols available for coccidia in reptiles.
Sulfonamides drugs have been a longstanding staple as a treatment tool for coccidia ever since the 1940s. Since then, numerous drugs have been developed for use against coccidia, but the sulfonamides persist to be one of the primary therapeutics applied to most species, including reptiles, they remain the most often approved therapy in reptiles, with sulfadiazine, sulfamethazine and sulfadimethoxine as the standard prescription (Walden, 2009). Nonetheless, anatomical, biological, behavioral, and biochemical differences in different reptile species should always be considered, in order to avoid therapeutic failures or serious, even fatal, occurrences.
In this respect, the present paper reports a mortal effect of a non-referenced therapy with sulfa drugs for coccidia in a youthful bearded dragon, prescribed by a reptiles’ non-experienced practitioner.
Case Description
A youthful bearded dragon (Pogona vitticeps) was referred to authors because of anorexia and lethargic status. A reptiles’ non-experienced vet practitioner had previously visited the bearded dragon, prescribing a non-accreditated therapy subsequently to a standard faecal flotation evidencing coccidia infection. Orally, a formulation (Disulfa, Formenvet s.r.l.) licensed in Italy for respiratory and enteric diseases of cage birds, was administered at the dose of 1 ml q 24 h for 7 days. The formulation is based on diaveridine 0.5% and sulfadimetoxine 0.5%, corresponding to an actual dosage schedule of 71.4 mg/kg for both drugs on a daily base. No advisement was given to the owner about the necessity to check for the hydration status of the bearded dragon, or the necessity to integrate fluids assumption during therapy because of the possible negative effect of sulfa drugs on urinary system. During the therapeutic cycle, the animal became progressively anorectic and lethargic, and at the end of the therapy it was referred to the authors. At admission, the bearded dragon was 23 cm length and 70 g weight, apathetic, moderately dyspnoic and seriously dehydrated, with pale dried mucous membranes and sunken eyes. A thermostatic cage was used for the hospitalization, with heating sets (40°C in the basking site and 29°C in the cold side) to warranty thermoregulation. It was not possible to collect samples for blood work because of the small size and the emaciated status of the animal, so supportive therapy was started considering the possibility of sulfa nephrotoxicosis on an anamnestic basis. Via an intraosseous catheter, placed in the tibial plateau anterior to the joint capsule after local anaesthesia with lidocaine (Perry and Mitchell, 2019), fluid therapy was administered. Fluids (1-part dextrose 5%, 1 part electrolytic rehydrating solution, 1 part saline, 1 part sterile water) at 3 ml/day were administered using a small syringe infusion pump. At 24 h since the admission, after a moderated correction of dehydration, assisted feeding (diluted Carnivore Care, Oxbow Enterprises, Inc.) at 1-2% b.w. was started, divided in 3 daily rations. After initial acceptance, tree day after admission the bearded dragon started regurgitation, so feeding was discontinued. In the same night the animal died.
In order to determine death causes, necropsy was performed according to the owner. At a gross examination (Figure 1), the abdominal cavity presented a moderate amount of sero-sanguineous fluid. Fat pads were normally developed. The liver was slightly enlarged with rounded margins, had a pale-yellow appearance, both on the capsule and cut surface, and was crumbly. Kidneys were discoloured and evenly moderately enlarged, while lungs appeared hyperaemic. Stomach and intestines looked normal. Samples of liver, kidney and lung were collected and fixed in 10% neutral buffered formalin (NBF), processed routinely and embedded in paraffin wax using an automatic tissue processor. Serial 5-μm sections from all specimens were cut with a 2030 Biocut microtome (Reichert-Jung, Germany and were mounted on glass slides (Super-Frost, Menzel-Gläser, Braunschweig, Germany). The sections were then stained with standard Haematoxylin and Eosin (H&E) and examined under a light microscope for detection of histopathological alterations.
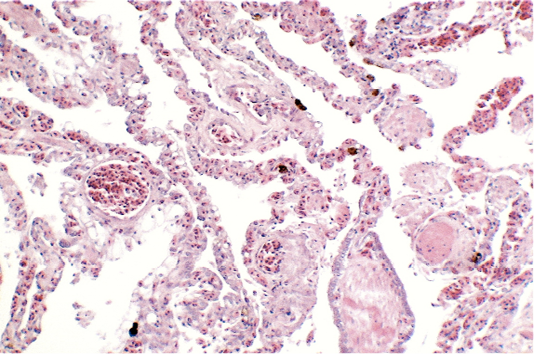
Renal samples (Figure 2) showed multifocal areas of coagulative necrosis, associated with marked marginal hyperaemia. The renal pattern showed interstitial nephritis, with presence of acidophilic mononuclear and polymorphonuclear cells. The examination of liver samples (Figure 3)
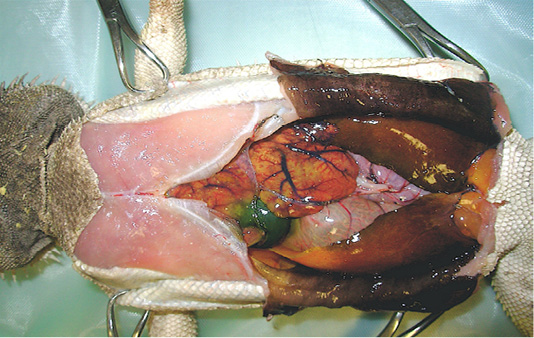
Figure 1: Necropsy examination of bearded dragon showing a moderate amount of sero-sanguineous fluid in abdominal cavity; a slightly enlarged liver with pale yellow appearance; a moderately enlarged and discoloured kidneys; and congested and hyperaemic lungs.
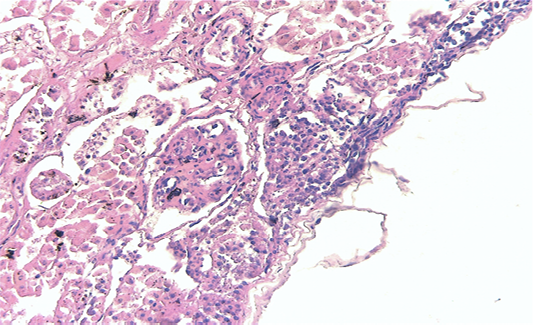
Figure 2: Kidney showing large multifocal areas of coagulative necrosis, associated with marked marginal hyperemia, interstitial nephritis, with presence of mononuclear and polymorphonuclear cells (H & E × 40, bar 100 μm).
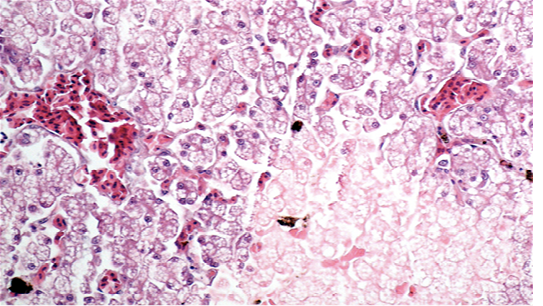
Figure 3: Liver showing cellular swelling and diffuse microvacuolar fatty change of hepatocytes, associated with scattered groups of necrotic hepatocytes; sinusoids were enlarged and congested (H & E × 20, bar 100 μm).
showed cellular swelling and diffuse fatty change of hepatocytes, associated with small scattered groups of necrotic hepatocytes. Sinusoids were enlarged and congested. Lungs showed areas of diffuse hyperaemia (Figure 4).
Discussion
Coccidia infections are a common finding in captive reptiles, with most of these infections being perpetuated because of poor hygiene of enclosures. Coccidia infections are typically self-limiting; however, autoinfection appears common in captive reptiles and can represent a serious risk for juvenile animals. Isospora amphiboluri may result in enteritis in heavy infections. Heavy numbers can also be associated with concurrent adenovirus (Atevirus, Adv) infections. This can lead to a wide range of clinical signs, including anorexia, lethargy, weight loss, diarrhea, tenesmus, prolapses, and death if untreated. Treatment of coccidia is recommended for young animals or those with a moderate to high parasite burden (Eatwell and Richardson, 2019).
Historically, sulfadimethoxine and trimethoprim-sulfamethoxazole have been recommended as coccidiostats for treating coccidia infections in reptiles. Unfortunately, these recommendations were mainly based on empirical data, especially for the posology, with little or no regard for species peculiarities. To date, there have been no pharmacokinetic studies performed to evaluate these drugs in reptiles. Current dosing recommendations are varied, including 15 to 25 mg/kg every 24 hours, 20 to 30 mg/kg every 24 to 48 hours, and 30 mg/kg once a day for 2 days then 30 mg/kg every 48 hours. The practitioner should remember that these doses are anecdotal and consider the general health status (mainly hydration status) of the patient before selecting a dose (Perry and Mitchell, 2019).
All sulfonamides work by interfering with the production of folate through competitive replacement of para-amino benzoic acid (PABA) in the structure of folate. The result of this competitive inhibition is a decreased amount of RNA and DNA synthesis and a resulting interference with metabolism, protein synthesis and growth of the parasite (Bowman et al., 1999; Boothe, 2001) These effects are not evident in the host since vertebrates lack dihydropterate synthase and must ingest folate from plants and animal prey (Murray, 1996). Sulfonamides are primarily effective against the asexual stages of coccidian development (merogony/schizogony), they operate as coccidiostatic drugs, and often prolonged treatment is required (Bowman et al., 1999). Potentiated sulfa drugs could be more fast effective. Trimethoprim, ormetoprim, pyrimethamine and diaveridine are usually used in combination with long acting sulfonamides: they synergize their anticoccidial activity by blocking the same metabolic pathway while exhibiting less host toxicity (Menholm and Armstrong, 2001).
Currently, there are several sulfonamides available, but sulfadiazine, sulfamethazine and sulfadimethoxine remain mostly recommended as the standard therapy for many species (Barnard and Upton, 1994). Sulfadimethoxine, one of the most widely used in reptiles, shares the same mode of action of other sulfonamides, but also the numerous disadvantages to using these compounds. The greatest inconvenience is the necessity for careful monitoring of hydration since sulfa drugs metabolites are excreted through the kidneys. Sulfadimethoxine is acetylated to acetylsulfadimethoxine in the liver of mammals and reptiles (Vree et al., 1989), and the metabolite excreted through the kidneys via both glomerular filtration and tubular secretion. It may crystallize in animals with concentrated urine causing crystalluria and urinary tract obstruction in mammals. The increasing of the dose can potentially enhance harmful side effects, and this possibility is still worse in desert species, which highly concentrate urine to spare water.
Many desert species can behaviorally conserve water by retreating to a humid underground burrow, but this may not be available in a cage with low relative humidity. In captivity, it is safest to assume all reptiles depend on environmental water. Some reptiles are not adept at drinking from water bowls: many terrestrial species (included bearded dragons) seem to prefer to walk into shallow water to drink or to have water dripped on their head (Boyer and Scott, 2019). Furthermore, the digestive system of bearded dragons is used in conjunction with the urinary system to prevent water loss: the urine flows into the cloaca and then it moves retrograde into the colon where the colon mucosa extracts more water and some salts before the concentrated urine is excreted with the faeces (Witten, 1993). In the present case, the possibility of adverse effect of sulfa drugs administration was announced by two concomitant occurrences: 1. the actual dose was higher respect to the recommendations in exotic medicine literature (McArthur, 2004; Perry and Mitchell, 2019); 2. the owner was not instructed about carefully check the hydrating status and water assumption in a desert species. The histopathological changes affecting the liver did not seem to be connected to the coccidia infection; also, the gallbladder was free of debris/gallstones and not enlarged, occurrence that would have suggested the previous presence of C. pogonae. Gross pathologic examination of reptiles that die with AdV infection can involve only the liver, which may be enlarged and have petechiae or pale areas scattered throughout, with hepatic necrosis histologically evident (Marschang, 2011). Unfortunately, no virologic test was performed for Advs in the present case.
Hepatic alterations were evident, but regrettably the small size and the clinical status of the animal hampered the possibility to perform any hematologic and biochemical evaluation, that would have been an indispensable diagnostic tool, so no information on hepatic parameters is available. The diversity of reptile species, their physiologic features, and the effects of intrinsic and extrinsic factors present unique challenges for accurate interpretation of the hemogram and biochemistry, but combining the clinical presentation with hematologic findings provides valuable information in the diagnosis and monitoring of disease, besides helping and guiding the clinician toward therapy and prognostic output (Stacy, 2011).
Literature is lacking of data about safety of use of diaveridine in reptiles, the low toxicity reported in mammals it is likely to hypothesize less or no participation in the final mortal toxic output.
Conclusions
Based on little or no species-specific pharmacokinetic records, drugs are used in reptile species. Most of the antibiotic dosage procedure for these animals are empirical or deduced from other species. Moreover , the development of a therapeutic treatment in reptile medicine depend by the broad range of anatomic, physiological and behavioural peculiarities of the various species enclosed in the Reptilia class, so cross-species deduction of drug dosage regimens from birds, mammals or even between reptiles species can be dangerous. The instruction for practitioners interested in exotic and reptile medicine practice is not to improvise therapeutic protocols, but rather to refer to specialised literature or more qualified colleagues.
Acknowledgements
The authors would like to thank Mrs. Rosa Leone for her technical help during this work.
Conflict of interest
The authors declare that they have no conflicts of interest.
authors contribution
All the authors equally contributed to the present study.
References