Advances in Animal and Veterinary Sciences
Research Article
Serosurvey of Peste des Petits Ruminants in Small Ruminants in the Hilly Terrain North-Eastern State of Sikkim in India
Vinayagamurthy Balamurugan1*, Bibitha Varghese1, Dhanavelu Muthuchelvan2, Kirubakaran Vinod Kumar1, Karuna Chettri3, Kuralayanapalya Puttahonnappa Suresh1, Gurrappanaidu Govindaraj1, Divakar Hemadri1, Parimal Roy1
1Indian Council of Agricultural Research–National Institute of Veterinary Epidemiology and Disease Informatics (ICAR–NIVEDI), Post Box No. 6450, Yelahanka, Bengaluru-560064, Karnataka, India; 2Division of Virology, Indian Veterinary Research Institute (IVRI) Campus, Mukteswar-263138, Nainital, Uttarakhand, India; 3Department of Animal Husbandry Livestock, Fisheries and Veterinary Services, Government of Sikkim, Krishi Bhawan, Tadong-737102, Gangtok, Sikkim, India.
Abstract | This cross-sectional serosurvey outlines the seroprevalence of peste des petits ruminants (PPR) in small ruminants in the hilly terrain north-eastern state of Sikkim in India. A total of 776 small ruminants’ (sheep [n = 99] and goats [n = 677]) serum samples were collected from 62 epidemiological units (epi-units) of the state using a two-stage sampling method from September 2017 to February 2018. The collected sera were screened for the small ruminant morbillivirus (SRMV) antibodies using a PPR-competitive enzyme-linked immunosorbent assay (cELISA). The results showed 1.16% (95% confidence interval [CI]: 0.61-2.19%) apparent prevalence with almost zero calculated true prevalence, which implies the insignificant prevalence of SRMV antibodies in the small ruminants population. Further, only a few samples from <5 % epi-units have shown positive for SRMV antibodies with an overall seropositive of <30% in the surveyed epi-units in Sikkim. This report is first of its kind on the seroprevalence of PPR in a unique niche of hilly terrain settings and it implies that SRMV has not yet become established in the small ruminants in Sikkim, despite the enzootic nature of the disease in the rest of India. This necessitates the imposing of strict quarantine measures and restrictions in the small ruminants trade along with the implementation of the intensive active surveillance programme to make a PPR free state.
Keywords | Cross-sectional study, Peste des petits ruminants, Seroprevalence, Small ruminants, Sikkim, India.
Received | October 12, 2020; Accepted | October 20, 2020; Published | November 15, 2020
*Correspondence | Vinayagamurthy Balamurugan, Indian Council of Agricultural Research–National Institute of Veterinary Epidemiology and Disease Informatics (ICAR–NIVEDI), Post Box No. 6450, Yelahanka, Bengaluru-560064, Karnataka, India; Email: balavirol@gmail.com
Citation | Balamurugan V, Varghese B, Muthuchelvan D, Kumar KV, Chettri K, Suresh KP, Govindaraj G, Hemadri D, Roy P (2020). Serosurvey of peste des petits ruminants in small ruminants in the hilly terrain north-eastern state of sikkim in india. Adv. Anim. Vet. Sci. 8(12): 1421-1426.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.12.1421.1426
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Balamurugan et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Peste des petits ruminants (PPR), is an acute, highly contagious, World Organisation for Animal Health (OIE) notifiable transboundary viral disease of sheep and goats and is caused by the small ruminant morbillivirus (SRMV), formerly known as peste des petits ruminants virus (PPRV). Clinically, the disease is characterised by high fever (pyrexia), oculo-nasal discharge, stomatitis, gastroenteritis, diarrhoea, and bronchopneumonia (Balamurugan et al., 2014b). The disease poses a heavy threat to the national economy of the enzootic countries (Balamurugan et al., 2014b) and it significantly impacts the small ruminants sector. After eradication of rinderpest, a global consensus on PPR Global Control and Eradication Strategy (GCES) has been reached on the need to control and eradicate the disease by 2030 (OIE and FAO, 2015). The Food and Agriculture Organization of the United Nations (FAO) and OIE launched an initial PPR Global Eradication Programme (PPR–GEP) for the period 2017–2021 to put the GCES into effect (OIE and FAO, 2015).
In India, PPR was first reported in 1987, and it continued to be present in Southern India until 1994 (Balamurugan et al., 2014b). Later, several PPR outbreaks were reported from the northern states (Singh et al., 2004a; Balamurugan et al., 2012) and the North-Eastern region (NER) since 2010 (Balamurugan et al., 2014a). In enzootic India, several outbreaks have occurred in the past and now are occurring throughout the country (Balamurugan et al., 2016) and cause major constraints in augmenting the productivity of small ruminants with an annual estimated financial loss of Indian rupees 16,110 million (Govindaraj et al., 2016). India initiated the national control programme for PPR (PPR-CP) using its resources based on the available epidemiological data, indigenously developed robust diagnostics, and vaccines to mitigate the disease burden. India practiced focused vaccination in outbreak places since 2002 and the strategic vaccination programme approach in some of the states (Balamurugan et al., 2016; Govindaraj et al., 2019) by launching national PPR–CP from 2010–2011 to control and eradicate PPR even before the global framework was established (Balamurugan et al., 2016). All the southern peninsular states and Union Territories (UTs) adopted strategic vaccination in the first phase, and the remaining states and UTs of India were included in the second phase from 2014-2015 (Balamurugan et al., 2016). However, some of the UTs and states including the unique niche of the archipelago, hilly terrains (Sikkim, Arunachal Pradesh) in the NER and other hilly states of northern India have not implemented the vaccination, due to the low incidence or no outbreaks of PPR experienced in these States or UTs (Balamurugan et al., 2019; 2020 b; c).
In NER, the first confirmed outbreak was recorded in Assam during 2010, and subsequently, different outbreaks were reported from Assam and Tripura states (Balamurugan et al., 2014a; De et al., 2016; Devi et al., 2016). Nevertheless, the majority of the reports from India represent only the regional isolated data using a limited number of samples (Singh et al., 2004a; Raghavendra et al., 2008; Balamurugan et al., 2011; 2014a; 2020 a; b; De et al., 2016; Devi et al., 2016). Furthermore, neither systematic post-vaccination monitoring nor any sero-surveillance or monitoring plan was undertaken except a few organized studies, that represents a zone or state/UT (Balamurugan et al., 2019; 2020b; c). The results from different studies in India demonstrated the widespread nature of the disease except for the Andaman Nicobar archipelago (Singh et al., 2004a; Raghavendra et al., 2008; Balamurugan et al., 2011, 2012; 2014a,c, 2016, 2019; 2020 a; b; c). The measurement of the prevalence of SRMV antibodies in different geographical locations with varying agro-climatic conditions is essential to develop PPR specific control strategies to the unique geographical location. Furthermore, prevalence study is of paramount importance to prevent disease infiltration especially in isolated geographical environments such as distant niche of the hilly terrain or archipelago as well as to acquire disease-free status by employing intensive active surveillance and monitoring programme. Therefore, a cross-sectional seroprevalence study in small ruminants in the hilly terrain settings of north-eastern Sikkim state of enzootic India was carried out from September 2017 to February 2018 to determine the SRMV antibodies status.
MATERIALS AND METHODS
Study Area
Sikkim is the second smallest state among the Indian states located in north-eastern India. It borders with Tibet in the north and northeast, Bhutan in the east and Nepal in the west, and West Bengal in the south. The state was purposively selected as it has a unique geographical location, and also not practiced PPR vaccination and not reported PPR outbreaks though India is enzootic to PPR. The village is discrete and considered as the epidemiological unit (epi-unit) in the studied area as reported (Balamurugan et al., 2019; 2020b).
Sampling Designs
The epi-unit level sample size estimation was determined by using a two-stage random sampling by using epi-calculator (https://nivedi.res.in/Nadres_v2/Epical/herd_level_sample_size.php) as described earlier (Balamurugan et al., 2020a). The number of secondary units (animal samples) within the village was calculated by the hypergeometric distribution as per GCES guidelines with the unit prevalence of 30 % (Singh et al., 2004a; OIE and FAO 2015) and the village (cluster) level prevalence of 5 % (Govindaraj et al., 2016; Govindaraj et al,. 2019) with 95% of target cluster sensitivity and confidence level. A total sample size of 62 primary units (villages) was determined with a maximum of 11 samples in each epi-unit with a maximum animal sample size of 682 were arrived for the infinite large-goat population. Further, a list of the villages and their sheep and goats population in the Sikkim was prepared based on the 19th Livestock Census, 2012 of India (Sikkim, has 113,364 goats and 2,634 sheep) and the villages having > 200 small ruminants (with inclusion and exclusion criteria) were shortlisted for the sampling frame. The estimated epi-units were proportionally allocated to the districts and distributed randomly in all the four districts of Sikkim in the generated sampling frame of 231 villages using R software (R_Core_Team 2014).
Samples
In the epi-unit, animals were selected by the simple random method, and in each surveyed epiunit, where only goats were present 11 samples from goats and in a village where both goats and sheep were present an additional 11 samples from sheep were collected. A total of 776 (sheep [n = 99] and goats [n = 677]) samples from 62 epi-units were collected through the collaborating centre of the Indian Council of Agricultural Research–National Institute of Veterinary Epidemiology and Disease Informatics (ICAR–NIVEDI), All India Coordinated Research Project on Animal Disease Monitoring and Surveillance (AICRP on ADMAS), Animal Husbandry & Veterinary Department, Tadong, Sikkim. The sample surveyed villages are depicted in the GIS map (Figure 1) based on their geo-coordinates using the open-source software QGIS (version 2.18.6, www.qgis.org). The collected blood sample was labelled and placed in a cool shipment box and transported to the laboratory for serum separation and the separated sera were transported to the ICAR–NIVEDI and stored at −20°C until further use.
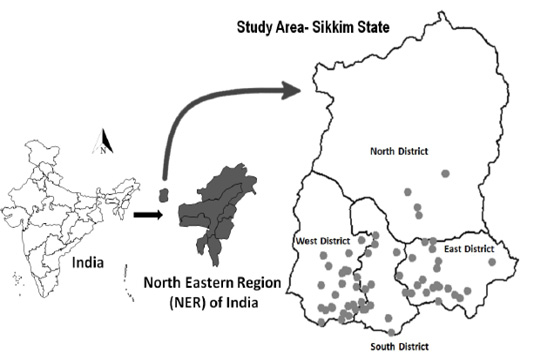
Figure 1: The surveyed epi-units (villages) are depicted (as ● dot) in the GIS map of Sikkim state in India
Testing and Analysis
The serum samples were tested by PPR competitive enzyme-linked immunosorbent assay (cELISA) for the detection of specific SRMV antibodies, which were measured in terms of percentage inhibition (PI) according to the protocol described by Singh et al. (2004b). Samples with a PI of ≥ 40% (Singh et al., 2004b) or ≥ 50% (Balamurugan et al., 2011) were considered as positive and the overall percentage positivity/prevalence was calculated. The apparent and true seroprevalence was estimated from the following formula described by Thrushfield (2005): Apparent prevalence = number of positive animals/numbers of tested animals. True prevalence = (apparent prevalence + [specificity − 1]) / ([sensitivity + specificity] − 1), which was calculated based on the relative (with the virus neutralisation assay) sensitivity (92.4%) and specificity (98.4%) of the employed c-ELISA (Singh et al., 2004b).
RESULTS AND DISCUSSION
On screening of the 776 serum samples, the observed apparent and calculated true seroprevalence was 1.16% and -0.005 %, respectively (Table 1). The district-wise details of the sera collected during the survey and its test results are summarised in Table 1. Only a few samples (n=9) from seven epi-units (Darap, Gyalshing, Phamthang, Paiyong (Kerabari), Navey, Luing, Nambu villages) have shown positive if PI cut-off set as ≥ 40%, whereas only four samples from two epi-units (Gyalshing and Nambu villages) have shown positive if PI cut-off set as ≥ 50%, with an overall, <30% seropositive animals in all the tested epi-units of Sikkim.
In Sikkim, goats, and sheep together constitute around 38% of the state livestock and over 65% of the rural poor landless farmers are widely rearing Shingharey goats, which are medium-sized and distinct from Black Bengal and other graded breeds (Anonymous 2020). Information on the prevalence of SRMV antibodies in different species has been reported from various enzootic countries (Almeshay et al., 2017; Acharya et al., 2018; Dayhum et al., 2018; Burns et al., 2019) including some studies from India with varying levels of seroprevalence (Singh et al., 2004a; Raghavendra et al., 2008; Balamurugan et al., 2014a; 2016; 2020a; b; Devi et al., 2016). The present study defines the prevalence of SRMV antibodies in small ruminants in Sikkim and generated evidence of a very low apparent seroprevalence, which might be due to the collected random samples were from apparently healthy animals and not from PPR suspected animals. Further, it also could be due to an ecological unique terrain and topology that restricts movements of animals from other regions, the low population density and availability of less grazing area leading to reduced transmission of the virus between animals, as close contact of small ruminants is needed for acquiring and spread of infection. Further, intermixing of these populations with sheep and goats from the rest of the country is usually limited because of the very narrow connecting geographical passage to the state.
The results from the study were concurrent with the earlier reported data from the other states in the NER including unique geographical areas having relatively less population density (Singh et al., 2004a; Balamurugan et al., 2014a; 2020b, De et al., 2016; Devi et al., 2016). The seroprevalence of less than 2.0 % has been reported in relatively low population density of small ruminants and geographically isolated hilly states (Himachal Pradesh, Assam, Meghalaya, and Tripura) and island (Andaman and Nicobar Islands) region of India (Singh et al., 2004a; Balamurugan et al., 2019). Further, Balamurugan et al. (2020b) reported that the prevalence of 34.3%, 10.3%, 4.7%, 15.7%, 14.7%, and 5.5% SRMV antibodies in small ruminants in Assam,
Table 1: District-wise details of samples tested and its results for small ruminant morbillivirus antibodies in sheep and goats in Sikkim
| Name of the district |
No. of the tehsil/
|
No. of village having >200 small ruminants included in the survey |
Targeted village as per proportion with 10% attrition rate |
No. of the village/ surveyed
|
No. of the samples collected and tested ( No. of samples positive)* |
Apparent Prevalence of SRMV antibodies % (CI-value at 95%) |
True Prevalence of SRMV antibodies
|
Seroprevalence of < 30 % in No. of epi-units |
||||||
|
To- tal |
She-ep | Goats | To-tal | She-ep | Goats | To-tal | She-ep |
Go- ats |
||||||
| East district | 3 | 66 | 20 | 20 | 231 (2) | 11 (0) | 220 (2) |
0.87 (0.24-3.11) |
0 (0-25.88) |
0.91 (0.25-3.25) |
-0. 008 |
-0. 018 |
-0. 008 |
20
|
| North district | 2 | 21 | 6 | 5 | 64 (0) | 11 (0) | 53 (0) |
0 (0-5.66) |
0 (0-25.88) |
0 (0-6.76) |
-0. 018 |
-0. 018 |
-0. 018 |
5
|
| South district | 2 | 66 | 20 | 13 | 175 (2) | 33 (1) | 142 (1) |
1.14 (0.31-4.07) |
3.03 (0.54-15.32) |
0.7 (0.12-3.87) |
-0. 005 |
0. 016 |
-0. 010 |
13 |
| West district | 2 | 78 | 24 | 24 | 306 (5) | 44 (0) | 262 (5) |
1.63 (0.7-3.76) |
0 (0-8.03) |
1.91 (0.82-4.39) |
0. 000 |
-0. 018 |
0. 003 |
24 |
| Total | 9 | 231 | 70 | 62 | 776 (9) | 99 (1) | 677 (8) |
1.16 (0.61-2.19) |
1.01 (0.18-5.5) |
1.18 (0.6-2.31) |
-0. 005 |
-0. 006 |
-0. 005 |
62 |
CI: confidence interval; SRMV: small ruminant morbillivirus; *Parenthesis: No. of the samples positive for SRMV antibodies.
Manipur, Meghalaya, Mizoram, Nagaland, and Tripura states, respectively with an overall 14.5% seroprevalence using stratified random sampling. All these studies, generally indicate the variation in the observed seroprevalence, which could be due to differences in the uniqueness of the geography or topology, sample size, sampling methods, prevailing animal husbandry and management practices, humidity, or season as reported earlier (Singh et al., 2004a).
From this study, it is possible to say (with 95% confidence) that fewer than 5% of villages out of 231 villages in the sampling frame in Sikkim have circulating PPR, but less than 30% antibody-positive animals in the epi-units. Nevertheless, the presence of SRMV antibodies in animals indicates that the population was exposed to SRMV infection naturally either directly or indirectly as the state has not practiced PPR vaccination. A few positive animals in Sikkim might be due to the introduction of earlier PPR vaccinated or recovered SRMV infected animals from the other geographical areas. Several published reports have generally indicated that most positive animals have migrated from neighbouring states (Singh et al., 2004a; Raghavendra et al., 2008; Balamurugan et al., 2011; 2012; 2014a;c; 2020b). These animals might have been brought by nomads or migrated shepherds from neighbouring states or through porous international borders of India in different phases of inhabitation and rehabilitation of migrated or settled shepherds. Therefore, to maintain the low prevalence or incidence levels or free status of PPR, transboundary migration of animals must be monitored in Sikkim and its border areas, which would be highly essential to protect this region. Since the susceptible small ruminants in Sikkim including some places in NER (Balamurugan et al., 2014a; 2020b; De et al., 2016; Devi et al., 2016) is naïve and there exists a high risk of acquiring SRMV infection if close contact happens with introduced infected animals from northern India or from the neighbouring states, where the disease is enzootic (Singh et al., 2004a; Balamurugan et al., 2014b; 2011).
Nonetheless, in this study, we could not examine prevalence variation due to unavailability of demographic data like sex, breed, flock dynamics, seasons, health status, husbandry practices, etc, and additionally, the target epi-units in the north and south Sikkim districts could not be covered due to administrative issues. FAO/OIE Global strategy for PPR eradication is generally based on understanding the epidemiological factors and risk-based vaccination coverage followed by surveillance. These baseline data are one of the primary requirements for disease control along with vaccine and diagnostic support in stage I of Global strategy. Now, India is in stage 2 (final phase), therefore, we have assessed only the prevalence status to plan the future strategy in the remote areas state for control and eradication of PPR.
This study is first of its kind from NER of Sikkim and reports all the sampled epi-units had < 30% seroprevalence and indicates the need for active surveillance in the state. Further, it also warrants appropriate control measures to prevent the spread of infection from the rest of India and bordering countries by implementing strict quarantine measures at the various animal entry places/points of the state, as well as imposing the legal framework like strict restrictions on the small ruminants movement and trade by allowing only if animals had vaccination history card and certifications by the competent/regulating authority.
CONCLUSION AND Recommendations
This study provides information on the seroprevalence of PPR in the unique geographically located hilly terrain Sikkim state in India with only fewer than 5% of villages in the state have circulating PPR but with <30% seropositive animals in the surveyed villages. It implies that the SRMV has not yet become established in the small ruminants in Sikkim and the state is naïve immune to PPR incursion from the rest of the enzootic states of India and entry from surrounding border countries. Hence, to protect the state from PPR, strict restrictions and quarantine measures need to be adopted along with the implementation of the active surveillance programme and legal framework. Furthermore, it is imperative for the intensive surveillance of sporadic outbreaks in different clinical forms of the disease, thereby control and eradication of the disease can be achieved and subsequently acquiring the disease-free status. Nevertheless, at the time of declaring India provisionally free from PPR, further prevalence/surveillance study of PPR in the Sikkim also needs to be carried out as per Global Control and Eradication Strategy sampling guidelines to support and demonstrate disease-free status in unvaccinated small ruminants in a unique hilly terrain north-eastern region of India.
ACKNOWLEDGEMENTS
The authors wish to thank the Indian Council of Agricultural Research (ICAR), New Delhi, India, for providing facilities and financial support of the Institute project (IXX14475) and the ICAR–National Institute of Veterinary Epidemiology and Disease Informatics (NIVEDI) staff for their constant support and encouragement always. The authors also thank the Investigators from, All India Coordinated Research Project on Animal Disease Monitoring and Surveillance (AICRP on ADMAS), Collaborating centre of ICAR–NIVEDI, Tadong, Sikkim for sending the serum samples to monitor the status of livestock diseases in Sikkim. The authors are thankful to the Department of Animal Husbandry Livestock, Fisheries and Veterinary Services, Government of Sikkim and their officials, field veterinarians, para-veterinarians for their assistance during samples or data collection.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
Compliance With Ethical Standards
Statement Of Animal Rights
The manuscript does not contain animal experimental trial. No ethical clearance is required for collecting small volumes of blood samples required for seroepidemiological studies, as per CPCSEA (Committee for the Purpose of Control and Supervision of Experiments on Animals) guidelines. Moreover, samples were collected by well-trained veterinarians concerning animal welfare regulations.
authors contribution
VB designed the work with overall monitoring, analyzed the data and drafted, and edited the manuscript. BV carried out the laboratory test. DM provided diagnostic support. KVK and GG analyzed the data and edited the manuscript. KC collection and transportation of the samples. KPS, DH designed the sample plan, managed the samples, and prepared GIS map for the study area. PR provided guidance and support in research work. All authors read, and approved the final manuscript.
REFERENCES





