Advances in Animal and Veterinary Sciences
Research Article
In-vitro Antimicrobial Activity and Adsorption Adequacy of Natural Clay against Some Air and Water Pollutants
Omnia F. Mohamed, Mahmoud M. Hussein, Essam S. Soliman*
Animal, Poultry, and Environmental Hygiene Division, Department of Animal Hygiene, Zoonosis, and Animal Behavior, Faculty of Veterinary Medicine, Suez Canal University, Ismailia 41522, Egypt.
Abstract | Clay is a natural outcome product of many environmental processes and has a wide diversity in its origins and end-uses in industrial and medical fields. The in-vitro antimicrobial activity of clay against E. coli O157: H7 and Salmonella typhimurium, as well as the adsorption capacity against air gaseous impurities like ammonia and water impurities including heavy metal, hardness, and organic matter salts were investigated. Three weights of clay (0.5, 1.0, and 2.0 g) were evaluated inside the laboratory against E. coli O157: H7 (1.5 x 108 CFU/ml), Salmonella typhimurium (3.5 × 105 CFU/ml), ammonia (80 ppm), lead nitrate (3.5 mg/L), magnesium sulfate (2160 mg/L CaCO3), and ammonium chloride (31.5 mg/L) using minimal inhibitory concentration (MIC) test at specified exposure intervals (5, 10, 20, 30, and 60 minutes). A total of 270 pollutant/contaminant-clay-air/water samples were collected (45 samples for each pollutant, 3 replicate × 3 clay weights × 5 interval times) and examined. The results revealed highly significant antimicrobial activities (P < 0.01) against E. coli O157: H7 and Salmonella typhimurium with reductions of the counts up to 84.7% and 47.6%, respectively when exposed to 0.5 g of clay for 60 minutes. Highly significant reductions (P < 0.01) were recorded in aerial ammonia concentrations, as well as, lead, hardness, and total organic matter water concentrations after exposure to 0.5, 2.0, 2.0, and 0.5 g of natural clay for 60 minutes with reduction percentages up to 4.5%, 41.7%, 41.6%, and 21.8%, respectively. The study concluded the highly efficient antimicrobial activities and adsorptive actions of natural clay concerning 0.5 and 2.0 g after an exposure time of 60 minutes.
Keywords | Aerial ammonia, Clay, E. coli and Salmonella, Hardness, Lead, Organic matter.
Received | September 04, 2020; Accepted | September 07, 2020; Published | November 15, 2020
*Correspondence | Essam S Soliman, Animal, Poultry, and Environmental Hygiene Division, Department of Animal Hygiene, Zoonosis, and Animal Behavior, Faculty of Veterinary Medicine, Suez Canal University, Ismailia 41522, Egypt; Email: soliman.essam@vet.suez.edu.eg
Citation | Mohamed OF, Hussein MM, Soliman ES (2020). In-vitro antimicrobial activity and adsorption adequacy of natural clay against some air and water pollutants. Adv. Anim. Vet. Sci. 8(12): 1367-1379.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.12.1367.1379
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Soliman et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
The air inside animal and poultry houses should be completely clean, a state that was defined latter as the freedom of the air from all forms of impurities that degrade the air quality and impair its functions (Auvermann, 2006). Many pollutants and impurities can gain access to the microclimatic air of animal and poultry houses and degrade its quality (Lim et al., 2004). These pollutants can arise from animal houses, manure storage facilities, and land application of manure. The rate of discharge and transmission of these pollutants dependent on microclimatic and macroclimatic temperature, relative humidity, ventilation rate, live-stock housing design, manure management systems, and host determinants as age, sex, breed, and stocking density. Air pollutants have a direct influence on the welfare, production, and reproduction of live stocks (Lai et al., 2012; Jacobson et al., 2011; Heber et al., 2006).
Water with optimum requirements and standard qualities should be provided for animals and poultry to prevent negative impacts that arise from water deprivation or improper qualities (Beede, 2009; Faries, 2007). Water is important to optimize ion exchange, lubrication, digestion, metabolism, thermoregulation, biological processes, and excretion (Hersom and Crawford, 2008). Water provided for the live-stocks has to be monitored regularly for many characters including temperature, pH, electrical conductivity (EC) expressed by μS/cm, total dissolved solids (TDS) expressed by mg/L, dissolved oxygen (DO) expressed by mg/L, hardness expressed by mg/L CaCO3, phosphate (PO42-) expressed by mg/L, sulfate (SO42-) expressed by mg/L, nitrate (NO32-) expressed by mg/L, electrolytes as sodium; potassium; calcium; magnesium and chloride expressed by mg/L, heavy metals as iron; copper; zinc and lead expressed by mg/L (Soliman et al., 2016), and bacterial and fungal counts as total Enterobacteriaceae count (TEC) expressed by CFU/mL (Soliman et al., 2009) and total bacterial (TBC) and fungal counts(TFC) expressed by CFU/mL (Soliman et al., 2009).
Clay is a natural product and outcome of many environmental processes, it can be produced by the breakdown of rocks and used in several applications (Guggenheim and Martin, 1995). Clay has a wide diversity in its origin and end-uses and this might explain its existence in different forms and accompanied by variable minerals (Churchman et al., 2006). Clay possesses several characteristics that make it suitable for the variable widespread uses, these characteristics like cations exchange abilities, catalytic activity, swollen appearance on moistening, hard texture when dried, fine-grained, and low permeability (García-Sanchez et al., 2002). Clay also possesses several chemical and physical characteristics as agglomeration, pelletizing, conditioning, de-dusting, coating, and roll compaction (Srinivasan, 2011).
The study aimed for determining the in-vitro antimicrobial activity of natural clay against E. coli O157: H7 (1.5 x 108 CFU/ml) and Salmonella typhimurium (3.5 × 105 CFU/mL), as well as, determining the in-vitro adsorptive efficiency of natural clay against some air gaseous impurities like ammonia gas (80 ppm) and some water impurities including heavy metal salts as lead nitrate (3.5 mg/L), hardness salts as magnesium sulfate (2160 mg/L CaCO3), and organic matter salts as ammonium chloride (31.5 mg/L).
MATERIALS AND METHODS
Ethical Approval
The methodologies and materials used in the current study were approved by the Scientific Research Ethics Committee, Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt with approval number (2020038).
Experimental Design
The in-vitro trials were designed for multifactorial randomized trials. The number of samples was calculated using a simple random sampling design (Thrusfield, 2005) with an expected error of 5% using the following formula:
n =1.962 Pexp (1 - Pexp) / d2
Where n = required sample size, Pexp = expected prevalence, and d = desired absolute precision.
Natural Clay
Clay was obtained from the sides of Ismailia Lake - Ismailia. The clay was sterilized by dry heat disinfection using a hot air oven (Daihan® LabTech® Hot air Oven, LDO-080N) at 160°C/25 min to ensure a state of complete freedom of microbial contamination. A specimen (1 kg) from the clay was used for chemical evaluation and sediment analysis. Clay was examined for some physical characteristics as recommended by Środoń (2006) and Nkoumbou et al. (2008) like pH, silt %, clay %, sand %, and texture, as well as, some chemical parameters as electrical conductivity (EC) expressed by dS/m, cations as recommended by Vaccari, (1998) like calcium (Ca2+); magnesium (Mg2+); sodium (Na+); potassium (K+) expressed by meq/l, anions as recommended by Vaccari, (1998) like carbonate (CO32-); aldehyde (HCO-); chloride (Cl-); sulfate (SO42-) expressed by meq/l, organic carbon (OC) expressed as %, organic matter (OM) according to Hamza et al. (2013) and expressed as %, and calcium carbonate content (CaCO3) expressed as %. The results of the chemical analysis were revealed in Figure 1.
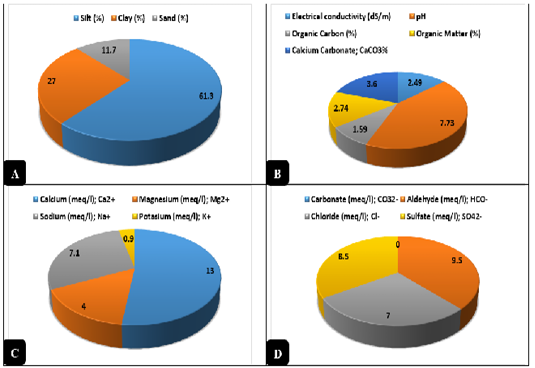
Figure 1: Physical and Chemical analysis of clay. A) Physical analysis of the natural clay (%). B) Chemical analysis of the natural clay. C) Cations contents of the natural clay expressed by meq/l. D) Anions contents of the natural clay expressed by meq/l.
Three weights of clay (0.5, 1.0, and 2.0 g) were examined using minimal inhibitory concentration (MIC) test against air gaseous impurities like ammonia gas and some water impurities including heavy metal as lead nitrate, hardness salts as magnesium sulfate, and organic matter salt as ammonium chloride, as well as against E. coli O157: H7 and Salmonella typhimurium.
Microbial Contaminants Propagation
E. coli O157: H7 2.6 × 105 CFU/ml suspension was provided willingly and free of charge from Animal Health Research Institute- Ismailia. The bacterial suspension was pre-enriched using Mac-Conkey broth (HIMEDIA, M-MacConkey Broth, M1125-500g) in tubes previously supplied with inverted Durham’s tubes and incubated at 44°C/24 hours as recommended by Soliman et al. (2018). Positive tubes exhibited the development of yellow color with gas accumulation in the inverted Durham’s tubes indicating the growth of pathogenic Enterobacteriaceae. Ten microliters (µL) from the positive tubes were dropped aseptically using the drop plate technique onto eosin methylene blue (HIMEDIA, Levine-Eosin Methylene Blue Agar, M022-500g) agar and incubated at 37°C/24 hours. Escherichia coli O157: H7 typical colonies that revealed metallic green appearance were counted and picked up until obtaining Escherichia coli O157: H7 1.5 x 108 CFU/ml suspension. Escherichia coli O157: H7 1.5 x 108 CFU/ml suspension was held into triplets of 15 ml capacity Falcon tubes at 4°C until used in the MIC test.
Salmonella typhimurium lyophilized vial (2.4 × 103 CFU) was purchased from Animal Health Research Institute - Dokki - Cairo. Salmonella typhimurium was propagated into tetrathionate broth (HIMEDIA, Tetrathionate Broth Base, w/o Iodine & BG, 500g) at 37°C/24 hours as recommended by Soliman et al. (2018). Ten µL from positive (turbid) tetrathionate tubes were dropped aseptically onto brilliant green agar (HIMEDIA Brilliant Green Bile agar, M059-500g) at 37°C/24 hours. Red and pink colonies of Salmonella typhimurium were counted, picked up, and reconstituted in buffered peptone water providing 3.5 × 105 CFU/ml suspension. Salmonella typhimurium 3.5 × 105 CFU/ml suspension was held into triplets of 15 ml capacity Falcon tubes at 4°C until used in the MIC test.
Air and Water Chemical Impurities and Stock Solutions Preparation
The impurities were purchased and prepared in-stock solutions of 1000 ppm of the element according to the following formula:
=(Molecular weight of the salt (g) × Required concentration of the element (g))/(Molecular weight of the element (g))
Ammonia commercial solution (30-33% NH3 in H2O, Merck, CAS Number 1336-21-6, Linear Formula NH4OH, Molecular Weight 35.05, 05002- 1L) was purchased and used as a source of aerial ammonia. Three conical flasks of one-liter capacity were exposed to ammonia solution (33-35%) and tested for the aerial ammonia concentration inside them, the procedures were repeated until the time of exposure contribute to an aerial ammonia concentration of 80 ppm inside the flask.
Lead nitrate powder (Lead di-nitrate, Merck, CAS Number: 10099-74-8, Linear Formula: Pb(NO3)2, Molecular Weight: 331.21, 1073980100- 100g) was purchased and used as a source of lead. Lead nitrate was added to each of three conical flasks contained one liter distilled water by a rate of 0.1 mg/L producing an average level of lead equal to 3.5 mg/L.
Magnesium sulfate powder (Magnesium sulfate anhydrous, Reagent Plus®, ≥99.5%, Merck, CAS Number: 7487-88-9, Linear Formula: MgSO4, Molecular Weight: 120.37, M7506- 500g) was purchased and used as a source of hardness. Magnesium sulfate was added to each of three conical flasks contained one liter distilled water by a rate of 0.075 mg/L producing an average level of total hardness equal to 2160 mg/L CaCO3
Ammonium chloride powder (Ammonium chloride for analysis EMSURE® ACS, ISO, Reag. Ph Eur, Merck, CAS Number: 12125-02-9, Linear Formula: NH4Cl, Molecular Weight: 53.49, 1011450500- 500g) was purchased and used as a source of organic matter. Ammonium chloride was added to each of three conical flasks contained one liter distilled water by a rate of 0.005 mg/L producing an average level of organic matter equal to 31.5 mg/L.
Minimal Inhibitory Concentration Test
Each weight of the clay (0.5, 1.0, and 2.0 g) was exposed independently to triplets of one-liter capacity flasks of aerial ammonia, water lead nitrate, water magnesium sulfate, water ammonium chloride solutions, and triplets of 15 ml capacity falcon tubes of Escherichia coli O157: H7 1.5 x 108 CFU/ml and Salmonella typhimurium 3.5 × 105 CFU/ml. At specified intervals (5, 10, 20, 30, and 60 minutes) specimens of the clay-pollutant/contaminate-air/water mixtures were harvested from each flask/tube to be tested.
Sampling
A total of 270 pollutant/contaminant-clay-air/water samples were collected (45 samples for each pollutant, 3 replicate × 3 clay weights × 5 intervals). E. coli and Salmonella-clay-water samples were subjected to tenfold serial dilution up to 106 into 15 ml capacity Falcon tubes and directed immediately for total Enterobacteriaceae and Salmonella counts. Ammonia-clay-air flasks were directed immediately for the chemical analysis of aerial ammonia, lead-clay-water samples were preserved by adding 0.5 ml nitric acid to each and sent to the toxicology laboratory for lead quantification, magnesium sulfate-clay-water samples were directed immediately for EDTA quantification of total hardness, and ammonium chloride-clay-water samples were directed immediately for estimation of organic matter.
Microbial Contaminants Examination
E. coli and Salmonella counts from the clay-pollutant/contaminate mixtures were conducted using the drop plate technique as recommended by Kim and Lee (2016) and Soliman et al. (2016). The mixtures were exposed to tenfold serial dilutions up to 106 as recommended by American Public Health Association; APHA (2017), then ten µl from each of the Falcon tubes were dropped onto eosin methylene blue (HIMEDIA, Levine-Eosin Methylene Blue Agar, M022-500g) for total Enterobacteriaceae count, and brilliant green agar (HIMEDIA Brilliant Green Bile agar, M059-500g) for Salmonella count, then the plates were incubated at 37°C/24 hours. Colonies revealed metallic green colors (E. coli) and red/pink colonies (Salmonella) were counted using the Dark-field colony counter (Quebec, Reichert-Jung) as recommended by Murray et al. (2015). The counts were transferred into logarithmic count using Microsoft Excel 2016. E. coli and Salmonella counts were expressed as CFU/mL. The detected logarithmic counts were compared to the stock count and reduction percentages at each exposure time were calculated.
Chemical Impurities Examination
Ammonia concentrations were quantified via potentiometric titration (American Public Health Association; (APHA, 2012) using sulfuric acid N/10 and titration against ammonium hydroxide in the presence of methyl orange as a pH color indicator, ammonia concentrations were expressed as ppm. Lead concentrations in the clay-pollutant/contaminate mixtures were quantified using atomic absorption spectrophotometer (APHA, 2012) and expressed as mg/L. Total hardness levels were quantified via traditional titration against EDTA solution 0.01 mol/L in the presence of aerochrom black-T as a pH color indicator (APHA, 2012), the hardness levels were expressed as mg/L CaCO3. Organic matter levels were quantified via titration against potassium permanganate 0.4 g/L in the presence of potassium permanganate 0.4 g/L and ammonium oxalate 0.8 g/L (APHA, 2012), the organic matter levels were expressed as mg/L.
The obtained levels of all chemical impurities were compared to the stock concentrations and the reduction percentages at each exposure time were calculated.
Statistical Analysis
Statistical analysis was carried out using statistical package for social sciences – SPSS version 24 (Green and Salkind, 2016 and IBM, 2016). The recorded results were analyzed using multifactorial two-tailed Analysis of Variance (ANOVA) to detect the adsorption activity of natural clay against air and water impurities (ammonia, lead nitrate, magnesium sulfate, and ammonium chloride) and the antimicrobial activity against E. coli and Salmonella typhimurium at different exposure times and their interactions. The overall means and the interactions were displayed in the illustrated tables. The statistical model was summarized as follow:
Yijk=µ + αi + βj + (αβ)ij + Ɛijk
Where Yijk was the measurement of the dependent variables; µ was overall mean; αi was the fixed effect of air and water impurities and contaminants; βj was the fixed effect of the exposure time; (αβ)ij was the interactions; Ɛijk was the random error. The bacterial counts were transferred into a logarithmic number (Log10) using Microsoft Excel 2016. Results were expressed for high significance at (P < 0.01), significant at (P ≤ 0.05) and non-significant at (P > 0.05)”.
RESULTS and DISCUSSION
Microbial Contaminants
Clay has been used in the ancient medical preparations for wound healing, diarrhea, dysentery, tapeworm infestations, hookworm infestations, putrefaction, fluxes, pain management, inflammations, and abscesses management (Williams et al., 2008). Natural clay contained nanoscale of less than 200 nm and illite-smectite, as well as, reduced iron and thus clay has been used for many years as an antibacterial agent in the modern medicinal preparations (Cunningham et al., 2010). Recently, clay has been used for healing wounds caused by Mycobacterium ulcerans. Clay also has high absorption power and can adhere to the skin offering dermatological protection against numerous physical and chemical agents (Tateo at al., 2009).
Clay revealed in Figure 2A highly significant antimicrobial activities (P < 0.01) against E. coli O157: H7. The logarithmic E. coli counts were greatly reduced when exposed to 0.5, 2.0, and 1.0 g of natural clay with reduction percentages up to 84.7%, 82.7%, and 75.4%, respectively from the original counts used in the challenge after 60 minutes of exposure. Meanwhile, Salmonella typhimurium counts revealed in Figure 3A highly significant declines (P < 0.01) as exposed to 0.5, 1.0, and 2.0 g of natural clay with reductions up to 47.6%, 27.7%, and 25.7%, respectively after 60 minutes of exposure. On a time scale, E. coli O157: H7 (Figure 2B) counts were greatly reduced (P < 0.01) up to 61.8, 74.8, 79.9, 88.2, and 100% after exposure to clay for 5, 10, 20, 30, and 60 minutes, respectively. Meanwhile, Salmonella typhimurium (Figure 3B) counts were greatly reduced (P < 0.01) up to 6.7, 10.4, 22.6, 58.2, and 70.5% after exposure to clay for 5, 10, 20, 30, and 60 minutes, respectively.
The interactions between the clay weights and the exposure times revealed highly significant declines (P < 0.01) in the mean values of E. coli O157: H7 (Figure 2C) and Salmonella typhimurium (Figure 3C) as the exposure times increases, while the interactions between the clay weights and the exposure times revealed highly significant increases (P < 0.01) in the reduction percentages of E. coli O157: H7 (Figure 2D) and Salmonella typhimurium (Figure 3D) as the exposure times increases.
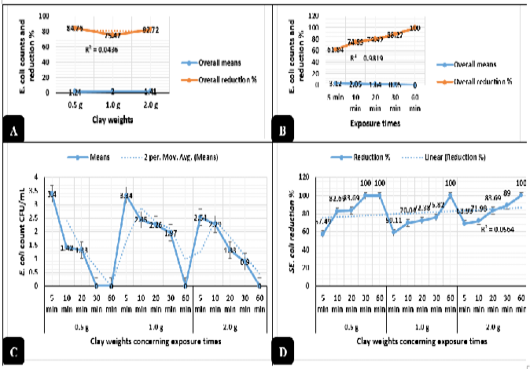
Figure 2: Antimicrobial action of natural clay on E. coli logarithmic counts during the in-vitro trials. A) Overall means of E. coli counts (CFU/mL) and reductions (%) concerning the clay weights. B) Overall means of E. coli counts (CFU/mL) and reductions (%) concerning the exposure times. C) E. coli counts (CFU/mL) concerning the clay weights and exposure times. D) E. coli reductions (%) concerning the clay weights and exposure times.
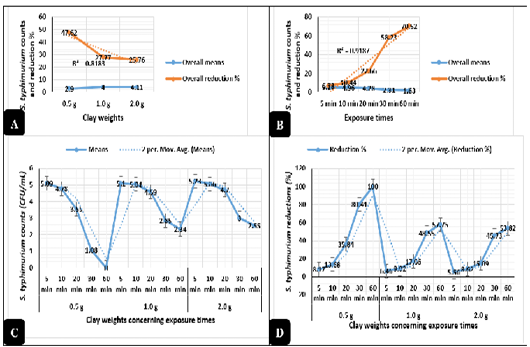
Figure 3: Antimicrobial action of natural clay on Salmonella typhimurium logarithmic counts during the in-vitro trials. A) Overall means of Salmonella typhimurium counts (CFU/mL) and reductions (%) concerning the clay weights. B) Overall means of Salmonella typhimurium counts (CFU/mL) and reductions (%) concerning the exposure times. C) Salmonella typhimurium counts (CFU/mL) concerning the clay weights and exposure times. D) Salmonella typhimurium reductions (%) concerning the clay weights and exposure times.
The results were consistent with those reported by Williams et al. (2011) who explained the broad-spectrum antibacterial actions of natural clay via the buffering of the pH and the oxidation process that enhanced the solubility of ferrous particles (Fe2+). They also stated that clay when exerted a bactericidal action on E. coli contributed elevations in the intracellular iron (Fe) and phosphorus (P) concentrations. The phosphorus takes a part in the control action of phospholipids over ferrous particles (Fe2+), these particles contradict the membranous proteins then oxidized into ferric particles (Fe3+) resulting in the formation of hydroxyl radicals which are lethal to the cell.
Ahmad et al. (2009) recovered the antibacterial actions of silver-clay-chitosan bionanocomposites (Ag-MMT-Cts, BNCs) against methicillin-resistant Staphylococcus aureus (MRSA) and gram-negative bacteria as Escherichia coli. They concluded the importance of the used composites in different biomedical fields. Otto et al. (2013) explored the antibacterial activity of clay against Escherichia coli and Staphylococcus aureus using x-ray diffraction, mass spectrometry, and optical emission spectroscopy to demonstrate clay leachates. They found positive correlations between clay leachates of high cobber (Cu2+), iron (Fe2+), and zinc (Zn2+) and the high antibacterial activity against Escherichia coli and Staphylococcus aureus. Behroozian et al. (2016) recorded an antimicrobial activity of clay against Enterobacter species, Staphylococcus aureus, Pseudomonas aeruginosa, Klebsiella pneumoniae, Enterococcus faecium, and Acinetobacter baumannii. They suggested the usage of clay against most of the diseases caused by the tested micro-organisms. Harrison et al. (2015) also recorded an antibacterial activity of clay against Staphylococcus aureus.
Chemical Impurities
Animal and poultry houses discharge large amounts of wastewater and waste materials that contained large amounts of ammonia contributed to environmental problems as eutrophication in water resources (Sun et al., 2015), as well as, affecting the water quality (Gupta and Nayak, 2012). Natural clay is known for its wide availability (Liu et al., 2017 and Xing et al., 2017), low cost (Franco et al., 2016), unique properties, and no toxic influences on the ecosystem (Ismadji et al., 2015 and Zhu et al., 2016). The unique properties of natural clay include an adsorption power for many gaseous and chemical elements concerning competitive adsorption and desorption actions against heavy metals including cadmium, chromium, cobber, lead, and zinc (Lukman et al., 2013) and dyes. Natural clay has
Table 1: Neutralization efficiency of natural clay against aerial ammonia concentrations (Mean ±SE) in in-vitro trials.
| Clay weights /g | Contact times /min | Ammonia ppm | Reduction % |
| Overall means in concern with Clay weights | |||
| 0.5 g |
76.4c±0.59 |
4.50a±0.04 |
|
| 1.0 g |
77.6b±0.28 |
2.96b±0.06 |
|
| 2.0 g |
78.1a±0.16 |
2.30c±0.02 |
|
| P-values | 0.000 | 0.000 | |
| Overall means in concern with contact times | |||
| 5 min |
78.8a±0.06 |
1.50d±0.08 |
|
| 10 min |
78.1b±0.11 |
2.38c±0.03 |
|
| 20 min |
78.0b±0.10 |
2.44c±0.03 |
|
| 30 min |
76.8c±0.43 |
3.88b±0.03 |
|
| 60 min |
75.1d±0.68 |
6.05a±0.05 |
|
| P-values | 0.000 | 0.000 | |
| Clay weights by contact times interactions | |||
| 0.5 g | 5 |
78.7a±0.13 |
1.66d±0.16 |
| 10 |
77.7b±0.02 |
2.83c±0.05 |
|
| 20 |
77.7b±0.01 |
2.83c±0.01 |
|
| 30 |
75.3c±0.01 |
5.83b±0.04 |
|
| 60 |
72.5d±0.09 |
9.33a±0.17 |
|
| 1.0 g | 5 |
78.9a±0.12 |
1.33d±0.04 |
| 10 |
78.1b±0.03 |
2.33c±0.07 |
|
| 20 |
78.2b±0.04 |
2.33c±0.05 |
|
| 30 |
77.0c±0.02 |
3.66b±0.14 |
|
| 60 |
75.8d±0.05 |
5.16a±0.08 |
|
| 2.0 g | 5 |
78.8a±0.00 |
1.50d±0.00 |
| 10 |
78.4b±0.00 |
2.00c±0.00 |
|
| 20 |
78.2c±0.11 |
2.16b±0.17 |
|
| 30 |
78.2c±0.08 |
2.16b±0.12 |
|
| 60 |
77.0d±0.01 |
3.66a±0.09 |
|
| P-values | 0.000 | 0.000 | |
Means carrying different superscripts in the same column are significantly different at (P ≤ 0.05) or highly significantly different at (P < 0.01).
Means carrying the same superscripts in the same column are non-significantly different at (P < 0.05).
SE= Standard error.
The stock value of aerial ammonia concentration is 80 ppm.
been used for its purifying qualities, filtration capacities, and purification of water from pollutants (Ghorbel-Abid et al., 2009, 2010; Ayari et al., 2007a, b).
Natural clay in Table 1 revealed highly significant decreases (P < 0.01) in aerial ammonia overall means up to 76.4, 77.6, and 78.1 ppm after exposure to 0.5, 1.0, and 2.0 g of clay for 60 minutes, respectively. The declines in overall means were accompanied by weak highly significant differences (P < 0.01) in the reduction percentages from the original ammonia load up to 4.5%, 2.9%, and 2.3%, respectively. On the time view, ammonia overall means values were subjected to highly significant decreases (P < 0.01), as well as, the reduction percentages revealed highly significant increases (P < 0.01) as the time of exposure increased, but the reduction percentages didn’t exceed 9% from the original aerial ammonia concentrations after exposure for 60 min to all weights of the natural clay. The interactions of the clay weights concerning different exposure times revealed highly significant decreases (P < 0.01) in ammonia values and highly significant increases (P < 0.01) of the reduction percentages of ammonia exposed to different weights of clay.
The results were consistent with those reported by Alshameri et al. (2014c) who recorded an increase in the ad
Table 2: Neutralization efficiency of natural clay against lead water concentrations (Mean ±SE) in in-vitro trials.
| Clay weights /g | Contact times /min | Leadmg/L | Reduction % |
| Overall means in concern with Clay weights | |||
| 0.5 g |
3.10a±0.05 |
11.42c±0.06 |
|
| 1.0 g |
2.85b±0.10 |
18.47b±0.07 |
|
| 2.0 g |
2.04c±0.07 |
41.71a±0.06 |
|
| P-values | 0.000 | 0.000 | |
| Overall means in concern with contact times | |||
| 5 min |
3.00a±0.03 |
14.28d±0.09 |
|
| 10 min |
2.78b±0.08 |
20.31c±0.07 |
|
| 20 min |
2.67c±0.07 |
23.49b±0.12 |
|
| 30 min |
2.71c±0.06 |
22.54b±0.02 |
|
| 60 min |
2.14d±0.01 |
38.73a±0.05 |
|
| P-values | 0.001 | 0.000 | |
| Clay weights by contact times interactions | |||
| 0.5 g | 5 |
3.36a±0.03 |
3.81d±0.05 |
| 10 |
3.20b±0.00 |
8.57c±0.00 |
|
| 20 |
3.10c±0.00 |
11.43b±0.00 |
|
| 30 |
3.10c±0.00 |
11.43b±0.00 |
|
| 60 |
2.73d±0.02 |
21.90a±0.03 |
|
| 1.0 g | 5 |
3.16a±0.01 |
9.52d±0.06 |
| 10 |
3.10b±0.00 |
11.43c±0.00 |
|
| 20 |
2.96c±0.00 |
15.24b±0.06 |
|
| 30 |
2.96c±0.01 |
15.24b±0.02 |
|
| 60 |
2.06d±0.02 |
40.95a±009 |
|
| 2.0 g | 5 |
2.46a±0.02 |
29.52d±0.03 |
| 10 |
2.06b±0.01 |
40.05c±0.03 |
|
| 20 |
1.96c±0.00 |
43.81b±0.01 |
|
| 30 |
2.06b±0.02 |
40.95c±0.05 |
|
| 60 |
1.63d±0.00 |
53.33a±0.03 |
|
| P-values | 0.000 | 0.000 | |
Means carrying different superscripts in the same column are significantly different at (P ≤ 0.05) or highly significantly different at (P < 0.01).
Means carrying the same superscripts in the same column are non-significantly different at (P < 0.05).
SE= Standard error.
The stock value of the water lead concentration is 3.5 mg/L.
sorption of ammonia on natural clay in a rapid manner and equilibrium was achieved within 30 minutes. He et al. (2016) stated that when the exterior surface of natural clay is saturated with ammonia, the ammonia ions started to enter through the pores and absorbed onto the interior surface of the particles. Alshameri et al. (2018) investigated six types of natural clay for their adsorption capabilities for ammonia. They found that the initial concentrations of ammonia, contact times, adsorbent dosage, natural clay structure, and surface properties affected the adsorption rate of ammonia. Soliman and Hassan (2017) reported that some natural compounds result from the natural reactions of clay with the surrounding might produce adsorption capabilities against ammonia when applied on the floors inside the poultry houses.
Lead nitrate in Table 2, revealed highly significant decreases (P < 0.01) in their overall mean concentrations (2.04, 2.85, and 3.10 mg/L) after exposure to 2.0, 1.0, and 0.5 g of clay for 60 minutes, respectively. The declines of lead overall means were synchronized with highly significant increases (P < 0.01) in the reduction percentages of the lead up to 41.7%, 18.4%, and 11.4% after exposure to 2.0, 1.0, and 0.5 g of clay for 60 minutes, respectively. The higher the exposure time, the greater the declines in the overall means, and the increases in the reduction percent
Table 3: Neutralization efficiency of natural clay against hardness levels in water (Mean ±SE) during the in-vitro trials.
| Clay weights /g | Contact times /min |
Hardness mg/L CaCO3 |
Reduction % |
| Overall means in concern with Clay weights | |||
| 0.5 g |
1404.0a±6.23 |
35.00c±2.78 |
|
| 1.0 g |
1350.0b±4.78 |
37.50b±2.07 |
|
| 2.0 g |
1260.0c±3.14 |
41.66a±1.57 |
|
| P-values | 0.002 | 0.000 | |
| Overall means in concern with contact times | |||
| 5 min |
1590.0a±5.40 |
26.38d±2.50 |
|
| 10 min |
1460.0b±2.91 |
32.40c±1.34 |
|
| 20 min |
1260.0c±2.12 |
41.66b±0.98 |
|
| 30 min |
1260.0c±0.00 |
41.67b±0.00 |
|
| 60 min |
1120.0d±2.17 |
48.14a±1.01 |
|
| P-values | 0.001 | 0.000 | |
| Clay weights by contact times interactions | |||
| 0.5 g | 5 |
1770.0a±5.00 |
18.05e±0.38 |
| 10 |
1530.0b±0.00 |
29.17d±0.00 |
|
| 20 |
1320.0c±7.00 |
38.89c±0.39 |
|
| 30 |
1260.0d±0.00 |
41.67b±0.00 |
|
| 60 |
1140.0e±8.00 |
47.22a±1.32 |
|
| 1.0 g | 5 |
1590.0a±2.21 |
26.39e±0.22 |
| 10 |
1500.0b±3.00 |
30.55d±0.33 |
|
| 20 |
1230.0d±2.15 |
43.05b±0.24 |
|
| 30 |
1260.0c±0.00 |
41.67c±0.00 |
|
| 60 |
1170.0e±0.00 |
45.83a±0.00 |
|
| 2.0 g | 5 |
1410.0a±3.02 |
34.72e±0.39 |
| 10 |
1350.0b±0.00 |
37.50d±0.00 |
|
| 20 |
1230.0d±2.01 |
43.05b±0.07 |
|
| 30 |
1260.0c±0.00 |
41.67c±0.00 |
|
| 60 |
1050.0e±2.55 |
51.39a±0.09 |
|
| P-values | 0.001 |
0.000 |
|
Means carrying different superscripts in the same column are significantly different at (P ≤ 0.05) or highly significantly different at (P < 0.01).
Means carrying the same superscripts in the same column are non-significantly different at (P < 0.05).
SE= Standard error.
The stock value of the water total hardness levels is 2160 mg/L CaCO3.
Table 4: Neutralization efficiency of natural clay against total organic matter levels in water (Mean ±SE) during the in-vitro trials.
| Clay weights/g | Contact times /min | Total organic matter ppm | Reduction % |
| Overall means in concern with Clay weights | |||
| 0.5 g |
24.62c±1.75 |
21.84a±0.57 |
|
| 1.0 g |
26.14a±1.30 |
17.01c±4.14 |
|
| 2.0 g |
25.18b±1.68 |
20.06b±0.34 |
|
| P-values | 0.002 | 0.000 | |
| Overall means in concern with contact times | |||
| 5 min |
26.70b±0.30 |
15.24b±0.97 |
|
| 10 min |
28.78a±0.24 |
8.60c±0.78 |
|
| 20 min |
28.78a±0.11 |
8.60c±0.01 |
|
| 30 min |
28.61a±0.09 |
9.17c±0.08 |
|
| 60 min |
13.67c±0.80 |
56.57a±2.54 |
|
| P-values | 0.000 | 0.001 | |
| Clay weights by contact times interactions | |||
| 0.5 g |
5 |
27.76b±0.13 |
11.85b±0.42 |
| 10 |
28.16a±0.35 |
10.53c±1.12 |
|
| 20 |
27.90b±0.23 |
11.43b±0.73 |
|
| 30 |
27.76b±0.12 |
11.85b±0.42 |
|
| 60 |
11.50c±0.10 |
63.49a±0.73 |
|
| 1.0 g | 5 |
26.16b±0.35 |
16.93b±0.12 |
| 10 |
29.36a±0.09 |
6.77c±0.42 |
|
| 20 |
29.23a±0.26 |
7.19c±0.84 |
|
| 30 |
29.23a±0.11 |
7.19c±0.42 |
|
| 60 |
16.70c±0.46 |
46.98a±1.46 |
|
| 2.0 g | 5 |
26.16c±0.35 |
16.93b±0.12 |
| 10 |
28.83b±0.48 |
8.46c±0.52 |
|
| 20 |
29.23a±0.04 |
7.19d±0.42 |
|
| 30 |
28.83b±0.35 |
8.46c±0.14 |
|
| 60 |
12.83d±0.31 |
59.25a±0.15 |
|
| P-values | 0.000 | 0.000 | |
Means carrying different superscripts in the same column are significantly different at (P ≤ 0.05) or highly significantly different at (P < 0.01).
Means carrying the same superscripts in the same column are non-significantly different at (P < 0.05).
SE= Standard error.
The stock value of the water total organic matter is 31.5 mg/L.
ages of lead nitrate concentration. The interactions of the clay weights concerning different exposure times revealed highly significant decreases (P < 0.01) in lead values and highly significant increases (P < 0.01) of the reduction percentages of lead-exposed to different weights of clay.
The results were synchronized with those reported by Chouchane et al. (2015) who stated that the usage of natural clay in the removal of heavy metals was more advantageous by simplicity and easy operation compared to traditional means including ion-exchange, precipitation, coagulation, oxidation, ozonization, and filtration. Oladipo and Gazi (2014), and Bentahar et al. (2017) stated that using clay for removing heavy metals like lead, chromium, and dyes from the water had become more efficient and economic. They attributed the high efficiency of natural clay in the removal of lead, chromium, and dyes for the small sizes of clay particles (<2 µm), high cations exchange capabilities, chemical stability, and high specific surface area. Ghorbel-Abid and Trabelsi-Ayadi (2015) stated that clay can be used as an efficient adsorbent for some heavy metals as chromium (Cr III) and Cadmium (Cd II) under optimized pH with an increase in the adsorption capacity in the single element system compared to binary-element system.
Water hardness can be attributed to the presence of calcium and magnesium ions and their salts in high concentrations in the water resources with a deleterious effect on the wellbeing of aquatic living and human (Wang and Lin, 2009). The use of hard water for animals and poultry, as well as, for the industry might contribute to extreme diseases for live beings and the pipe line in the industry (Hammer & Hammer, 2005). Hardness level in water during the in-vitro trials revealed in Table 3, highly significant declines (1260 mg/L CaCO3, P < 0.01) after 60 minutes exposure to 2.0 g natural clay compared to the other experimental groups. The declines in hardness levels were accompanied by highly significant increases in the reduction percentage of hardness levels up to 41.6% after 60 minutes of exposure to 2.0 g natural clay. The reductions in hardness levels were recorded at greater levels as the time of exposure increased. The higher reduction percentage recorded was 51.3% after exposure of water samples to 2.0 g clay for 60 minutes. The results were consistent with those recorded by Apell and Boyer (2010) who revealed high and promising adsorption capabilities of clay for calcium and magnesium ions contributing water hardness. Lijalem (2015) and Aveen and Kafia (2014) explained the adsorption capabilities of clay to cation exchange and the bonding of oxygen to form larger molecules.
Natural clay 0.5 g revealed in Table 4, highly significant decreases (P < 0.01) in the overall means of the total organic matter up to 21.8% after 60 minutes of exposure compared to other samples exposed to 1.0 and 2.0 g of clay. Based on the exposure times, the greater the time of exposure, the higher the declining degree in the total organic matter values up to 13.67 ppm out of 31.5 ppm, and the greater the reduction percentage in the total organic matter values up to 56.57%. The interaction of clay weights concerning different exposure times revealed that the highest reduction percentage of the total organic matter recorded was 63.4% in water exposed to 0.5 g of clay after 60 minutes.
Natural clay has been used for the treatment and removal of organic matter from ground and wastewater as recommended by Li et al. (2015), Bel Hadjltaief et al. (2016), Bel Hadjltaief et al. (2018), and Bel Hadjltaief et al. (2019). They contributed these abilities for the ability of clay to act as a heterogeneous photo-Fenton catalyst via the high iron content producing hydroxyl groups that didn’t have any negative impact on the water or the environment. The use of clay as a catalyst in the treatment of water and wastewater has been categorized as green chemistry for the absence of any pollutants that may result from the reactions. Aghamohammadi et al. (2007) and Hassani et al. (2008) reported that natural clay in the form of a powder or even granules has great abilities for the adsorption of organic materials from water. Djebbar et al. (2012) found that activated clay exhibited great potentials compared to natural clay for the removal of phenolic compounds from water. They also recorded that natural clay was able to reduce the phenolic compounds from the water up to 60% from their original concentrations. Aragaw and Ayalew (2018) found that clay can successfully remove hardness contributed to calcium and magnesium salts in water. They recorded that natural clay can remove calcium by a rate of 37.2% to 94.1%, and magnesium by a rate of 22.5% to 81.4% from water at the same temperature.
CONCLUSION
The current study revealed highly efficient antimicrobial activities of natural clay concerning 0.5 g against E. coli O157: H7 1.5 x 108 CFU/ml and Salmonella typhimurium 3.5 × 105 CFU/mL with reductions in the logarithmic counts up to 84.7% and 47.6%, respectively after 60 minutes of exposure.
Natural clay revealed also high adsorption efficiencies concerning 0.5 g against aerial ammonia concentration and water total organic matter with reductions up to 4.50% and 20.0%, respectively, as well as, high adsorption efficiencies concerning 2.0 g against water lead and hardness levels with reductions up to 41.7% and 41.6% after 60 minutes of exposure.
The concluded actions can be directed and applied in a large scale water treatment using clay filters to minimize microbial contaminants and chemical pollutants like hardness, organics, and heavy metals. Also, clay can be directed for the application on the floors inside poultry farms to control the emission of ammonia.
ACKNOWLEDGMENTS
Sincere thanking should be provided to Prof. M.A.A. Sobieh for his tremendous directions during the experiment.
Conflict of interest
The authors declare they have no competing interests.
AUTHORS’ CONTRIBUTION
ESS designed the experimental design, participated in the execution of the experiment, and writing of the manuscript. OMF participated in the execution of the experiment, and writing of the manuscript. MMH supervised the execution of the experiment and writing of the manuscript.
REFERENCES
Nasrollahzadeh Saravi H, Ghafari Sh (2007). Performance of a powdered activated carbon (pac) augmented activated sludge process treating semi-aerobic leachate. Int. J. Environ. Res. 1:96–103.
systems by natural clay: Adsorption and photo-Fenton degradation processes. Comptes Rendus Chimie. 21: 253-262. https://doi.org/10.1016/j.crci.2017.01.009
ternary systems with natural clay: Kinetic, isotherm, and thermodynamic. J. Environ. Chem. Eng, 5: 5921-5932.
Remediation. Volume 25 Springer, Surabaya. https://doi.org/10.1007/978-3-319-16712-1
percutaneous migration of chemical elements from a thermal mud for healing
use. Appl. Clay Sci. 44: 83–94.





