Advances in Animal and Veterinary Sciences
Research Article
Egyptian Propolis 16: The Effect of Consumption of Propolis and Alginate-Propolis Nanoparticles in Combination with Colostrum on the Performance of Newborn Goats
Walid M.A. Sadek1, Asmaa S. El-Houssiny2, Ayman Al-Mwafy1, Tarek Korany Farag3*, Ahmed Al-Gethami4, Sara I.M. Grawish1, Ahmed G. Hegazi5
1Sheep and Goat Research Department, Animal Production Research Institute, Agriculture Research Center, Dokki-Giza, Egypt; 2Microwave Physics and Dielectric Department, National Research Centre, Dokki-Giza, Egypt; 3Parasitology and Animal Diseases Department, National Research Centre, Dokki, Giza, Egypt; 4Al Guthami Foundation, Saudi Arabia; 5Zoonotic Diseases Department, National Research Centre, Dokki-Giza, Egypt.
Abstract | The aim of this study is to assess the possibility of using Propolis and Alginate-propolis nanoparticles (ALg-propolis NPs) to improve the health status of newborn Egyptian Nubian kids as a natural additive or as a replacement of colostrum to avoid the increasing rate of mortality under natural the system. Alginate ALg NPs were prepared by the controlled gellification method. Morphological analysis of the Alg-propolis NPs was examined using a Transmission Electron Microscope (TEM). Fifty twins Egyptian Nubian goats (Zaraibi) kids were randomly allotted and divided into five groups, 10 in each group. The rearing systems during the suckling period which extended to 13 weeks are as follow: C: Free suckling (FS), where the born kids were kept with their dams till being 13 weeks old (control). T1: (FS) + 0.6 ml propolis (twice/week). T2: (FS) + 0.0 6 ml ALg-propolis NPs (twice/week). T3: Artificial suckling on 100% goat’s milk in the first week (AS) + 0.6 ml propolis (duplicate dose after birth) then (FS) till being 13 weeks old + 0.6 ml propolis (twice/week). T4: (AS) + 0.06 ml ALg-propolis NPs (duplicate dose after birth) then (FS) + 0.06 ml Alg-P NPs (twice/week). The kids were fed either by mother feeding or artificial feeding on colostrum and supplied with propolis or ALg-propolis. The kids were weighted biweekly and the daily body weight gains were recorded. The kids’ body weight, milk consumption, and milk chemical were analyzed. Blood was collected for hematological parameter and serum biochemical analysis. Also, the frequency of diarrhea and dehydration were observed during the critical period in multiply births under the natural suckling system. The TEM study revealed that the ALg-propolis NPs are discrete and have spherical shapes with average particle size of 24 ± 17 nm. It was observed that, the groups treated with propolis and nano-propolis with and without colostrum accelerated the weaning period. Also it is interesting to find that the groups treated with Alg-propolis NPs without colostrum have a higher body weight than the groups treated with propolis alone (10.70 ± 0.90 and 10.03 ± 0.89 kg respectively) even though with its low dose. Moreover, the ALg-propolis NPs decreased the frequency of diarrhea during the 15 days of life as compared to pure propolis (0.27 ± 0.14 and 0.40 ± 0.19) respectively. Additionally, the milk consumption was slightly reduced with the nano formulation. Also, the results indicated that the blood parameters such as (RBCs, total protein, albumin, and globulin) were slightly increased with both Propolis and nano-propolis without statistical significance (P<0.05). Hence, these results indicated that the nano-encapsulation of Propolis within Alg NPs improved the health status of kids even with its low dose. Therefore, the results obtained suggest the feasibility of developing a successful propolis oral delivery nanosystem on an industrial scale using the ALg NPs. Moreover, they were recommended to be used as a replacement of colostrum to improve the performance of newborn kids.
Keywords | Suckling goat, Propolis, Alginate, Nano-propolis, Diarrhea
Received | July 29, 2020; Accepted | September 08, 2020; Published | November 15, 2020
*Correspondence | Tarek Korany Farag, Sheep and Goat Research Department, Animal Production Research Institute, Agriculture Research Center, Dokki-Giza, Egypt; Email: tarek.korany@yahoo.com
Citation | Sadek WMA, El-Houssiny AS, Al-Mwafy A, Farag TK, Al-Gethami A, Grawish SIM, Hegazi AG (2020). Egyptian propolis 16: The effect of consumption of propolis and alginate-propolis nanoparticles in combination with colostrum on the performance of newborn goats. Adv. Anim. Vet. Sci. 8(12): 1256-1265.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.12.1256.1265
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Sadek et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Newborn ruminants have three critical periods related to their immune system development during the first two months of their life: Colostrum feeding, milk feeding, and weaning. Management during these periods affects the final animal performance (Htoo et al., 2015). Moreover, small ruminants fed an inadequate amount of colostrum in the first hours of life . Therefore, they are more susceptible to disease and mortality (Hernández-Castellano et al., 2015; Polizel et al., 2016). Colostrum and milk contain fat, proteins, lactose, and minerals, which are all of nutritional importance (Rashid et al., 2012). Besides, they contain vitamins, immunoglobulins, hormones, growth factors, cytokines, enzymes, and bioactive peptides (Kuralkar and Kuralkar, 2010). (Nowak and Poindron, 2006). The consumption of colostrum by the progeny of ruminant species (cow, sheep, and goat) has a fundamental role in the passive immune transfer and the survival rate of newborns (Hernández-Castellano et al., 2015).
Egyptian Nubian (Zaraibi) goats are considered of high genetic potential as dairy and prolific breed (Aboul-Naga,1993). Hamed et al. (2009) reported that 17.1% of the Zaraibi herd in El-Serw experimental station gave birth only of one kid, whereas multiple births were recorded in 82.9% of total goats (twins: 55.7%; triple: 30% and quadruplets: 4.2%). Alimentary tract diseases manifested in a form of diarrhea are the most common health problems in kids during the first weeks of their life. Etiology of diarrhea is complex, except environmental and nutritional factors; infectious factors are an important cause of that disease (Sunderland et al., 2003). Many authors have found high mortality of newborn kids, with natural suckling, ranged from 30.8 to 65 % in desert goats (Salama, 1993) and reached 25 % in Baladi goats (Gihad et al., 1985), while did not exceed 18 % in Zaraibi goats as reported by Abdelhamid et al. (1999). Moreover, an increased rate of losses in twin kids of Zaraibi goats were recorded (Abdelhamid et al., 2011; Ahmed et al., 2013).
Propolis is a natural brownish-green resinous product which collected by the honeybees from the buds of trees. Propolis was being used to make the protective shield at the entrance of the beehive (Silva-Carvalho et al., 2015). Propolis has been used since ancient times as a medicine (Hegazi, 2012) because of its biological properties as an antimicrobial (Hegazi et al., 2014, 2019), antifungal (Kujumgiev et al., 1999), antiprotozoan (Hegazi et al., 2018), antiparasitic (Hegazi et al., 2007) and antiviral agent (Kujumgiev et al., 1999; Hegazi et al., 2012; Fan et al., 2013), immunostimulating (Takagi et al., 2005). Propolis contains a range of biologically active compounds like phenol compounds, flavonoids (primuletin, chrysine, tecochrysine, akacetine, galangine, morin, robinetin), terpenes, lipid-wax substances, bioelements, vitamins (A, D, F, K, E, B1, B2, B5, B6, B12, C), enzymes (alpha and beta amylase), amino acids, sterols, steroids, plant steroids and plant sterols (ergosterol, stigmasterol, steroidal saponins, steroidal alkaloids) (Sahinler and Kaftanoglu, 2005).
Alginate polymer (Alg) is a naturally occurring polysaccharide found in all species of brown algae (Repka and Singh, 2009). Alginate NPs has been found in many biotechnological and biomedical applications in view of its several advantages such as high biocompatibility, biodegradability and non-toxicity (Szekalska et al., 2016). They are well known for their controlled drug release properties and used for the encapsulation of many active pharmaceutical agents (Sarei et al., 2013). ALginate-propolis NPs are nano-sized (~1–100 nm in diameter) particles which are tried to make the propolis more effective (Seven et al., 2018). ALg-propolis NPs have better efficacy in the fields of medical science and biology (Afrouzan et al., 2012). Animal production and nanotechnology are important areas of research and development (Seven et al., 2018). However, there is not yet enough common use of nanotechnology in the market (Vance et al., 2015).
Natural products are an alternative for chemotherapeutic agents (Zhang et al., 2018), especially when the phenomenon of microorganism’s resistance to antibiotics is developed (Azzam et al., 2017).
Thus, the aim of this study was to improve the potency of propolis by encapsulating it within ALg NPs Moreover, it was aimed to improve the health status of newborn Egyptian Nubian (Zaraibi) kids by using propolis or ALg-propolis NPs as a natural additive or as a replacement of colostrum.
MATERIALS AND METHODS
Propolis
Propolis sample was collected from the apiary farm near El-Mansoura City, Dakahlia Governorate, Egypt. The resinous materials were kept in a dark bag in the refrigerator until being extracted with ethanol. Sodium Alginate was supplied by ROTH, Germany. CaCl2 was supplied by Qualikems, India. The materials used are with analytical grade.
Extraction and sample preparation
Fifty grams of propolis samples were cut into small pieces and extracted at room temperature with 500 ml of 70% ethanol (twice after 72 hours). The alcoholic extract was evaporated under vacuum at 50oC until dryness (Hegazi et al., 2019). The locally prepared propolis was previously evaluated for their phytochemical and biological analysis by (Hegazi et al., 2007). The percentage of the extracted sample is 5.1 g/dry weight.
Preparation of ALg NPs and propolis-ALg NPs samples
Alginate nanoparticles (ALg NPs) were prepared by a controlled gellification method based on the ionotropic gelation of polyanion with CaCl2 (Rajaonarivony et al., 1993). Alginate solution with concentration (0.1% w/v) was obtained by dissolving the polymer in distilled water at room temperature. Then, 5 ml of CaCl2 solution (36 mM) was added dropwise under constant stirring to 95 ml of ALg solution to induce gellification. The nanoparticles obtained were stirred for three hours at room temperature. For propolis-loaded ALg NPs sample, 2 ml of propolis with concentration (5mg/ml ethanol) was mixed with the ALg solution for twenty-four hours before CaCl2 addition. This mixture was further stirred for three hours at room temperature. Finally, the nanoparticles were freeze-dried then stored (El-Houssiny et al., 2016, 2017).
Transmission electron microscopy (TEM)
The morphological analysis of the ALg-propolis NPs was examined using a Transmission Electron Microscope (TEM) (JEM- HR- 2100 microscope operated at 120 KV, Japan). A sample of the nanoparticle’s suspension was dropped onto the copper grid. After complete drying, the sample was stained using phosphotungstic acid (El-Houssiny et al., 2016, 2017).
Experimental treatments
Ethical approval
All experimental procedures were performed in accordance with the institutional guidelines of the National Research Centre’s Animal Research Committee under protocol number 16/219.
Animals
This investigation was conducted at the Animal Production Research Station; Agriculture Research Center, El-Serw, and National Research Centre, Egypt. Fifty twins Egyptian Nubian kids were randomly allotted to one of the five treatments ten in each group which extended to 13 weeks as follow:
C: Free suckling (FS), where the born kids were kept with their dams until being 13 weeks old (control).
T1: (FS) + 0.6 ml propolis (twice/week).
T2: (FS) + 0.06 ml ALg-propolis NPs (twice/week).
T3: Artificial suckling (AS) on 100% goat’s milk in the first week + 0.6 ml propolis (duplicate dose) then (FS) till being 13 weeks old + 0.6 ml propolis (twice/week).
T4: (AS) on 100% goat milk in the first week + 0.06 ml ALg-propolis NPs (duplicate dose) then (FS) till being 13 weeks old + 0.06 ml ALg-propolis NPs (twice/week).
The groups which allowed to natural suckling were reared with their dams for 24/d from born to weaning with giving propolis and ALg-propolis NPs twice/week. While two group kids were separated from their dams and reared artificially in the first three days on 100% goat milk with giving propolis and ALg-propolis NPs to avoid consuming the colostrum, then kept with their dams to weaning (Ahmed et al., 2003; Htoo et al., 2015). The kids were kept in separate cages to facilitate proper management. Starter and berseem hay were available post the first 4th weeks of suckling. The kids were weighed biweekly of life and the daily body weight gains in that period were determined on that basis. An assessment of clinical symptoms of diarrhea, dehydration, and vitality of kids (reductions in severity of clinical signs on days 4 and 10 compared to day 0, and between days 4 and 10, was observed (Sunderland et al., 2003).
Milk composition and consumption
Milk samples were taken from the goat dams of all groups in the morning and evening, and then all week’s samples were analyzed for chemical composition (total solids (TS), fat, protein, solids non-fat (SNF) and Ash (Costa et al., 2014).
Blood parameters
Clinically all kids’ groups were monitored for hematological and biochemical parameters. Two blood samples were collected from the jugular vein once before feeding (3 animals from each group) at the end of the suckling period. Blood was collected into an evacuated tube containing Ethylenediaminetetraacetic acid (EDTA) for hematological parameter determination (Dini et al., 2015). The blood samples were analyzed in terms of erythrocyte (RBCs) and leukocyte counts (WBCs), hemoglobin (Hb) concentration, hematocrit (HCT), mean corpuscular hemoglobin concentration (MCHC), mean corpuscular volume (MCV), mean cell hemoglobin (MCH) values and platelet counts. The coagulated blood samples were centrifuged at 4000 rpm for 20 min. The separated serum was stored frozen until the biochemical analysis (Total protein, Albumin, Globulin, Creatinine, AST, ALT) (Allaoua and Mahdi, 2018).
Statistical analysis
Data were statistically analyzed using SAS (SAS Institute 2003).
RESULTS AND DISCUSSION
Transmission electron microscopy (TEM)
Particle size is one of the most important physicochemical characteristics of NPs. A small nanoparticles size can affect cellular interactions with biological systems. It can enhance the cellular uptake, efficacy and bioavailability of the active drug compounds in a formulation (Hegazi et al., 2019). (Gaumet et al., 2008). The particle size and surface morphology of the ALg-Propolis NPs were examined by a transmission electron microscopy; Figure 1. It shows that the NPs have small particle size in the nanometer range with an average particle size of 24 nm ± 17 nm. Moreover, the TEM view showed that the NPs are spherical in shape and discrete with smooth surface. Therefore, the TEM results suggested that the ALg-propolis NPs can permeate through cell membranes more effectively and are stable in the blood stream due to its small particle size. Thus, this may be reflected on the health status of newborn kids and may enhance their performance.
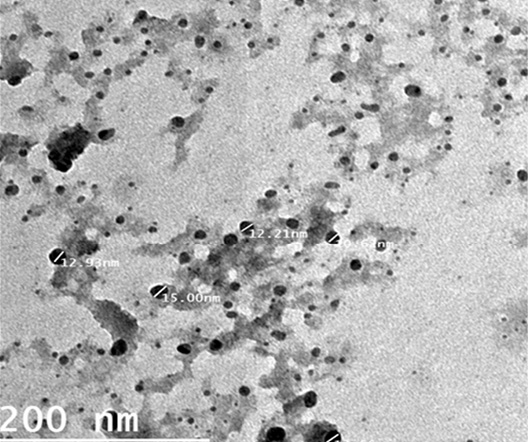
Figure 1: TEM micrographs of ALg-propolis NPs.
Kid’s performance
Milk composition
The chemical compositions of goat’s milk of the different experimental treatments are summarized in Table 1. Analysis showed insignificant differences in milk compositions among the five treatments. The obtained values of milk constituent are within the normal range given by Shehata et al. (2007), Abdelhamid et al. (2011) and Ahmed et al. (2011) for Egyptian Nubian goats. However, fat % was slightly decreased with the free suckling treatments C, T1 and T2 (3.72 3.71 and 3.73 %, respectively) compared with the artificial suckling in the first week T3 and T4 (3.75 and 3.77, respectively). The same results were obtained by Ahmed et al. (2013) who reported that the natural suckling system had significantly more average daily milk yield than artificial suckling system especially during the first weeks of lactation and consequently milk fat percentage was decreased. Similar results were observed also by El-Feel and Marzouk (1998). Moreover, Delgado-Pertíñez et al. (2009) reported that both milk fat and total solids percentage were affected by the suckling systems (natural or artificial).
Table 1: The chemical composition as % of goat’s milk of the different experimental treatments ten in each group.
| Items | C | T1 | T2 | T3 | T4 |
| Fat | 3.72 | 3.71 | 3.73 | 3.75 | 3.77 |
| Protein | 2.86 | 2.85 | 2.84 | 2.81 | 2.80 |
| Lactose | 4.61 | 4.64 | 4.61 | 4.62 | 4.60 |
| Total solids | 11.86 | 11.85 | 11.85 | 11.87 | 11.87 |
| Ash | 0.67 | 0.65 | 0.67 | 0.69 | 0.70 |
No symbols subscripted means insignificancy of difference among means. C: Free suckling (FS), where born kids were kept with their dams till being 13 wks old (control); T1: (FS) + 0.6 ml propolis (twice/week); T2: (FS) + 0.06 ml Alg-propolis NPs (twice/week); T3: (AS) in first week + 0.6 ml propolis (duplicate dose) then (FS) till being 13 wks old + 0.6 ml propolis (twice/week); T4: (AS) + 0.06 ml Alg-P NPs (duplicate dose) then then (FS) till being 13 wks old + 0.06 ml Alg-propolis NPs (twice/week).
Milk consumption
The data in Figure 2 show the effect of treatments on the milk consumption by kids. It was noticed that the milk consumption was slightly increased with (T1 and T2, respectively) than the other groups. However, this trend continued till the fourth weeks of suckling then differences were insignificant (P˂0.05). Meanwhile, in T3 and T4 groups, the consumption of milk was decreased till the fourth week. This may be due to the artificial suckling in the first week of these groups as reported by Ahmed et al. (2003, 2013).

Figure 2: The effect of using propolis and ALg-propolis NPs on the daily milk consumption of newborn Egyptian Nubian kids.
It is also interesting to find that after the 6th week, the
milk consumption shifted to be more with the C group than the other groups, then as weaning became close, start at week 8th, milk consumption backed to be more either with T3 and T4 vs. T1 and T2 or with colostrum vs. without colostrum milk. It was noticed that the feed intake of both starter and berseem hay consumed by treated groups was increased parallel to a decrease of milk suckled. In addition, it was noticed that 3 to 4 of the kids stopped suckling during the last two weeks
Table 2: Body weight changes of newborn Egyptian Nubian kids as affected by different experimental treatments.
| Item | C | T1 | T2 | T3 | T4 |
| No. of does | 5 | 5 | 5 | 5 | 5 |
| No. kids | 10 | 10 | 10 | 10 | 10 |
| No. of weaning kids | 8 | 10 | 10 | 9 | 10 |
| Birth weight, kg | 1.83±0.10 | 1.90±0.10 | 1.93±0.07 | 2.07±0.07 | 1.93±0.07 |
| Weaning weight, kg |
9.63±0.53b |
14.18±0.90a |
13.15±0.39a |
10.03±0.89b |
10.70±0.90b |
| Total body gain, kg |
7.80±0.52b |
12.28±0.83a |
11.23±0.33a |
7.97±0.92b |
8.78±0.91b |
| Daily body gain of kids, g |
92.86±6.19b |
146.13±9.85a |
133.63±3.90a |
94.84±10.92b |
104.46±10.87b |
| Mortality rate % | 20 | 0 | 0 | 10 | 0 |
a, b Differences between the groups are significant statistically (mean ± SE) on the level of (P<0.05).
(11th and 13th week). Accordingly, the treatment with propolis or ALg-propolis NPs with or without colostrum seemed to encourage the Egyptian Nubian kids to be early weaned.
Body weight changes and mortality rate
Body weights of kids were measured biweekly of different suckling groups as shown in Figure 3. In general, narrow differences were noticed among C, T3 and T4 groups in body weights and all kids had the same trend of increase by time. While, the differences were significant (P<0.05) with T1 and T2 vs. other groups. The highest body weight at 12w of life was noted in T1 and T2 (14.18 + 0.90 and 13.15 + 0.39 kg, respectively) followed by T4 and T3 (10.70 + 0.90 and 10.03 + 0.89 kg, respectively) as shown in Table 2. The lack of daily gains in group C may be explained by the fact that in several kids’ moderate diarrhea symptoms were noted at the first 2 months of life. As a result, these kids consumed smaller amount of starter feed. Similar results had been obtained by Robert et al. (2012) who found that the preventive application of 10 % ethanol extract of propolis (EEP) improved the health status of the calves in the neonatal period. After an application of propolis in a dose of 4 ml/day, higher daily gains were noted when compared to the control calves.

Figure 3: The effect of using propolis and Alg-propolis NPs on the body weights of newborn Egyptian Nubian kids (biweekly management, kg).
The mortality rate was with the T3 group (10%) compared with the C group (20%) while in the rest treatments, the percentage was (0%). This result may be due to the increasing incidence of diarrhea and dehydration in groups C compared with T1, T2, T3 and T4 groups as a positive effect of using propolis or Alg-propolis NPs on the bacteria caused diarrhea in the first 15d as a critical period for the newborn goats Table 3. On the basis of the results obtained with Hegazi and El-Hady (2001) who reported that the propolis showed the highest antimicrobial activity against Staphylococcus aureus, Escherichia coli and Candida albicans. Relatively good antimycotic activity was previously identified in the Egyptian propolis by Hegazi et al. (2000).
Table 3: Effect of treatments on the frequency of diarrhea, dehydration and vitality cases in newborn Egyptian Nubian kids during the critical period (according to Sunderland et al., 2003 in an own modification).
| Groups | Diarrhea | Dehydration | Overall condition |
| 15 day of life | |||
| C | 0.40±0.15 | 0.41±0.10 | 0.39±0.21 |
| T1 | 0.18±0.05 | 0.22±0.18 | 0.20±0.12 |
| T2 | 0.23±0.13 | 0.25±0.16 | 0.21±0.20 |
| T3 | 0.40±0.19 | 0.32±0.15 | 0.28±0.15 |
| T4 | 0.27±0.14 | 0.30±0.12 | 0.22±0.11 |
| 30 day of life | |||
| C |
0.42±0.03a |
0.38±0.08a |
0.31±0.10a |
| T1 |
0.17±0.07b |
0.22±0.05b |
0.18±0.11b |
| T2 |
0.15±0.09b |
0.20±0.04b |
0.16±0.11b |
| T3 |
0.18±0.05b |
0.21±0.03b |
0.17±0.12b |
| T4 |
0.13±0.03b |
0.20±0.09b |
0.16±0.11b |
a, b Differences in the same column are significant statistically (mean ± SE) on the level of (P<0.05). C: Free suckling (FS), where born kids were kept with their dams till being 13 wks old (control); T1: (FS) + 0.6 ml propolis (twice/week); T2: (FS) + 0.06 ml Alg-propolis NPs (twice/week); T3: (AS) in first week + 0.6ml propolis (duplicate dose) then (FS) till being 13 wks old + 0.6 ml propolis (twice/week); T4: (AS) + 0.06 ml Alg-propolis NPs (duplicate dose) then then (FS) till being 13 wks old + 0.06 ml Alg-propolis NPs (twice/week).
Ahmed et al. (2013) reported that the mortality rate was least with the artificial suckling on goat milk (9.09%) then the suckled mixed milk (13.04%) and the highest in the suckled cow milk (17.39 %) due to the increasing incidence of digestive disturbances (diarrhea and blot). Literature gave higher mortality of born kids, with natural suckling, ranged from 30.8 to 65 % in desert goats (Salama, 1993; Thiruvenkadani and Karunanithp, 2007) and reached 25% in Baladi goats (Gihad et al., 1985), while did not exceed 18% in Zaraibi goats as reported by Abdelhamid et al. (1999).
Clinical examination
The frequency of diarrhea, dehydration and vitality cases in newborn Egyptian Nubian (Zaraibi) kids during the critical period are presented in Table 3. No typical, acute clinical symptoms of alimentary tract disorders were observed during the whole research period. The highest diarrhea intensity was noted at the 15 day of life in kids from experimental groups. An application of the preparation of propolis or ALg-propolis caused a significant (P<0.05) decrease in a point-scale assessment of diarrhea intensity at the 30 days of life. At the 30 day of life, an overall condition of kids and also dehydration assessment, were more advantageous in kids that were given both type of the preparation. In this respect, Ahmed (1999) observed that the incidence of diarrhea in newborn kids was higher during the first weeks of suckling period and ranged from 20 to 28 cases according to the nutrition system. The application of the propolis and ALg-propolis NPs caused a significant (P<0.05) decrease in a point-scale assessment of diarrhea intensity at the 30 days of life (Mujica et al., 2017). Morsy et al., 2013 observed that the propolis has a good impact on the ewe’s health and parasitic response and it may be a promising feed additive during a critical period.
Moreover, Ahmed et al., 2013 observed that an increased incidence of diarrhea in the suckling Zaraibi kids during the first 21 days then a slight increase occurred in the last week before weaning. Soltan et al. (2015). The result of this investigation regarding decreased incidence of diarrhea and dehydration in first 15d as a critical period for newborn which may be due to the positive effect of Propolis or ALg-propolis which have antibacterial activity.
Table 4: Effect of experimental treatments on some hematological and serum biochemical parameters of Egyptian Nubian goats.
| Items | Groups | |||||
| C | T1 | T2 | T3 | T4 | ||
| Hematological parameters | ||||||
|
RBC's, ×106/µl |
8.46±0.17 | 8.53±0.18 | 8.46±0.16 | 8.96±0.07 | 10.03±0.78 | |
| Hb, g/dl |
14.56±0.64b |
13.92±0.10b |
19.68±0.78b |
22.6±0.64ab |
23.03±0.10a |
|
| HCT, % | 126.33±0.95 | 131±0.32 | 138±1.48 | 181.33±0.95 | 187.66±0.32 | |
| MCV, fl | 0.86±0.03 | 0.87±0.04 | 0.85±0.01 | 0.81±0.01 | 0.87±0.04 | |
| MCH, pg |
0.06±0.03a |
0.06±0.02a |
0.05±0.02ab |
0.04±0.01ab |
0.02±0.01b |
|
| MCHC, g/dl | 6.76±0.48 | 7.26±0.35 | 5.15±0.49 | 5.13±0.23 | 4.45±0.31 | |
|
Total leucocytic count, ×103/µl |
14.83±1.12 | 13.63±1.00 | 15.83±1.01 | 12.86±1.12 | 16.06±1.00 | |
| Neutrophils, % | 54.66±1.15 | 51.00±0.88 | 56.33±0.33 | 54.33±1.15 | 56.67±0.88 | |
| Lymphocytes, % | 37.33±0.88 | 40.33±1.45 | 34.66±0.88 | 38.00±0.68 | 35.66±1.45 | |
| Monocytes, % | 5.00±0.58 | 5.33±0.31 | 4.66±0.67 | 5.66±0.58 | 4.67±0.33 | |
| Eosinophils, % | 3.17±0.33 | 3.13±0.30 | 2.73±0.27 | 3.07±0.23 | 2.93±0.36 | |
| Basophils | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
|
Platelet count, ×103/µl |
234.66±11.67 | 231.33±8.82 | 235.66±18.12 | 227.66±11.67 | 236.66±8.82 | |
| Serum biochemical | ||||||
| Total protein, g/dl | 7.00±0.12 | 7.20±0.15 | 8.00±0.12 | 8.07±0.19 | 8.20±0.15 | |
| Albumin, g/dl | 2.73±0.26 | 2.83±0.13 | 2.73±0.26 | 2.97±0.12 | 2.83±0.13 | |
| Globulin, g/dl | 4.27±0.33 | 4.37±0.28 | 5.27±0.33 | 5.10±0.21 | 5.37±0.28 | |
| Creatinine | 0.99±0.01 | 0.84±0.05 | 0.97±0.07 | 0.83±0.09 | 0.87±0.07 | |
| AST, U/L |
17.67±0.67a |
16.66±0.88b |
16.65±0.67b |
15.17±0.63b |
14.67±0.88b |
|
| ALT, U/L | 159.03±10.27 | 159.06±18.84 | 160.04±10.27 | 152.03±4.98 | 151.05±18.84 | |
| ALT/AST | 8.99±0.48 | 9.54±1.36 | 9.05±0.48 | 10.01±0.34 | 10.29±1.36 | |
a, b Means in the same row with different superscripts differ significantly (mean ± SE) at P < 0.05.
In this study, it was observed that the groups treated with ALg-propolis NPs without colostrum (T4) exhibited less incidence rate of diarrhea during the 15 and 30 days of life (0.27 ± 0.14 and 0.13 ± 0.03) than the groups (T3) treated with pure propolis (0.40 ± 0.19 and 0.18 ± 0.05) respectively. Therefore, this indicates that the nano-propolis formulation is more effective than pure propolis as it enhances the health status of the kids by decreasing the incidence rate of diarrhea.
Blood parameters
The data in Table 4 show the effect of propolis (0.6ml) and ALg-propolis NPs (0.06ml) with or without colostrum on the hematological and serum biochemical parameters of Egyptian Nubian newborn kids. The RBC’s were slightly increased without significant for the groups treated with propolis and nano-propolis as compared to control. While, the hemoglobin concentration was significantly increased with the groups treated with propolis and ALg-propolis NPs (T2, T3 and T4) compared with the C group. On the contrary the MCH value was significantly decreased. Moreover, there were no significant differences observed for the total leucocyte (WBC) and differential leucocytes count compared with control.
Additionally, it was observed that the propolis and nano-propolis increased (P<0.05) the total protein and globulin without significant but AST reduced significantly (P<0.05) compared with control. In this respect, (Cetin et al., 2010b) reported the effect of 4 different levels of propolis supplementation (0.5, 1, 3, and 6 g of propolis/kg of diet) on the hematological and immunological parameters of laying hens. He observed that, the level of 3 g/kg diet of propolis increased (P<0.05) RBC compared with the other treatments while, hemoglobin, hematocrit values, total leucocyte (WBC) and differential leucocytes counts were not influenced by propolis supplementation. The same results were observed by Cetin et al. (2010a) who demonstrated that administration of propolis alone (100 mg/kg BW/day), does not cause any significant alteration on the hematological parameters (RBC, Hb, PCV, WBC and white blood cells proliferation) and biochemical indices including (AST, ALT, and Total protein) in female Wistar–Albino Rats blood. Morsy et al., 2013 indicated that Brazilian red propolis (BRP) administration increased (P < 0.01) TP and globuline. Propolis inhibitory effects on lymph proliferation may be associated to its anti- inflammatory property. In immunological assays, the best results were observed when propolis was administered over a short-term to animals (Sforcin, 2007).
CONCLUSIONS AND RECOMMENDATIONS
The obtained results suggest the feasibility of developing a successful propolis oral delivery nano system on an industrial scale using Alg NPs. Moreover, it recommends the usage of nanoparticles with Propolis as a good replacement of colostrum to avoid any shortage in kids’ requirements especially weakness of mothering ability by increasing the twinning rate so that improved the performance of newborn goat kids.
Acknowledgments
This study was financially supported by the National Research Centre, Giza, Egypt, with a grant no. 12070104. All authors have contributed significantly and that all authors are in agreement with the content of the manuscript is included.
Author’s Contribution
Walid M.A. Sadek: Collect and grouping of Kids, Preparing the Feeding for kids, Observation of animals, Did the statistical analysis, Help in righting of the article
Asmaa S. El-Houssiny: Preparing the the ALg nanopaticles encapsulation of propolis within ALg NPs, Characterization of the NPs, Discussion of the results, Writing the paper. Ayman Al-Mwafy: Observation of animals, Health care of animals, Weighting of animals and made blood sampling. Tarek Korany Farag: hematological parameter, serum biochemical analysis. Treatment of diarrhea,Treatment of dehydration and wrote the discussion related to study in the original manuscript draft. Ahmed Al-Gethami: prepared the tables and the figures, Discussion of the results, Help in publication. Sara I.M. Grawish: Performance of blood parameters, Counting RBCs, Determination of total protein, albumin, and globulin. Ahmed G. Hegazi: prepared the original idea, Designer of the experiment, Coordinatization of the team, Preparation and extraction of the propolis conceptualized the aim, design, plan of the study, Discussion of the results and wrote and revising the original manuscript draft. All authors contributed to revising the final draft of the manuscript. All authors read and approved the final manuscript
Conflict of interest
The authors have declared no conflict of interest.
REFERENCES





