Advances in Animal and Veterinary Sciences
Research Article
Effect of Lactobacillus-Based Probiotic in the Prevention and Complex Therapy of Brucellosis
Nina Nicolaevna Gavrilova*, Amankeldi Kurbanovich Sadanov, Irina Alexandrovna Ratnikova, Ainur Islamovna Seitbattalova, Batyr Abdikarimovich Kulnazarov
LLC, Research and Production Center for Microbiology and Virology, Bogenbai Batyr Street, 105, Almaty, 050010, Kazakhstan.
Abstract | The aim of the current study is to evaluate the effect of the lactobacillus-based probiotic with antagonistic activity against various species of brucellae on laboratory animals in the prevention and complex therapy of brucellosis. Our results revealed that the usage of probiotics for preventative and therapeutic purposes reduced the infection index and the bacterial load in the internal organs of outbred nonlinear white mice infected with a highly virulent strain Brucella melitensis 16M. Thus, the index of infection of internal organs in animals that received the probiotic before infection for 5 days decreased compared to the control by 25%, in animals that received the probiotic after infection for 20 days - by 58.75%. The greatest therapeutic effect was revealed with the combined use of a probiotic with the rifampicin antibiotic for 10 days. In this group of animals, no animals infected with brucellae were found after the end of treatment. In the group of animals that took only an antibiotic for treatment, the infection index was 1.3%.
Keywords | Probiotic, Lactobacillales, Brucellosis, Prevention, Therapy
Received | August 01, 2020; Accepted | August 7, 2020; Published | August 28, 2020
*Correspondence | Nina Nicolaevna Gavrilova, LLC, Research and Production Center for Microbiology and Virology, Bogenbai Batyr Street, 105, Almaty, 050010, Kazakhstan; Email: gavrilova.n.n@inbox.ru
Citation | Gavrilova NN, Sadanov AK, Ratnikova IA, Seitbattalova AI, Kulnazarov BA (2020). Effect of lactobacillus-based probiotic in the prevention and complex therapy of brucellosis. Adv. Anim. Vet. Sci. 8(s3): 18-22.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.s3.18.22
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Gavrilova et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
In the modern world, the problem of brucellosis is still very important. The causative agents of this dangerous zoonotic virulent disease persist and circulate in extensive hot spots with periodic epizootic outbreaks. Infected human cases are characterized by a tendency for a chronic relapsing course, leading to prolonged sickness absence and disability (Berger, 2016). Most often, human lesions are caused by B. melitensis represented by 3 biovars (the main hosts are sheep and goats). Less often human lesions are caused by B. abortus represented by 9 biovars (the main host is cattle), and by B. suis represented by 4 biovars (the main hosts are pigs, hares, and reindeer). In rare cases, human lesions are caused by B. canis (the main host is dogs). A significant epidemic danger of mixed brucellosis foci in farms of joint keeping of small ruminants and cattle under conditions conducive to the migration of B. melitensis to cattle has been established (Kalinovskiy, 2006).
According to the World Health Organization, the disease is detected in more than half a million people every year in 100 countries (WHO, 2006).
Human of any age is susceptible to the disease and majority of cases, people get infected as a result of eating meat and dairy products of diseases domestic animals or being in contact with them (care, feeding, slaughter, and other). So, this can determine how is the spread of brucellosis throughout the world, especially in countries with developed animal agriculture (Kuznetsov et al., 2011).
Brucellosis treatment requires a long term complex therapy which depend mainly of causal antibacterial therapy. In addition to causal therapy, patients are prescribed non-steroidal anti-inflammatory medicine, antihistamines, vitamin B complex, and physiotherapy (Kim et al., 2013). Prevention and control of brucellosis should be based on a set of veterinary-sanitary and medical-sanitary measures that aimed for reduction and elimination of the brucellosis incidence in farm animals (Kim et al., 2013).
According to previously obtained data, one of the ways to increase the effectiveness of brucellosis control in animals and humans can depend on the usage of Lactobacillales with high antagonist activity against the brucellosis causative agents (Gavrilova et al., 2003, 2006).
A comparative study of the antagonistic activity of twenty lactobacilli strains against five reference strains of Brucella (Brucella melitensis 16m, B. abortus 544, B. suius 1330, B. ovis 066, B. neotomae) revealed that the efficacy of six active strains of lactobacilli (Lactobacillus brevis B-3, L. salivarius 8d, L. plantarum 17, L. fermentum 7n, 175, and 27).
Moreover, these lactobacilli strains were active against all Brucella strains and the values of activity slightly fluctuated with a minimal inhibitory concentration of 1: 1,000 (L. brevis B-3, L. fermentum 7n) and 1:10,000 (L. salivarius 8d). The antagonistic activity of the L. salivarius 8d strain was also earlier determined on white mice that were subcutaneously infected with B. abortus 544 in comparison with the gentamicin antibiotic (Gavrilova et al., 2003).
The previous studies showed that the infection index and the bacterial load in internal organs (compared with the control) were significantly lower in groups, in which animals received Lactobacillales for five days before infection with Brucella or within 20 days after infection, and in the group, in which mice were treated with gentamicin antibiotic for ten days starting on the 20th day after infection. Therefore, the antagonistic activity of the L. salivarius 8d strain in vivo against Brucella is equivalent to the gentamicin effect (Gavrilova et al., 2003).
Then, the therapeutic and preventive efficacy of L. salivarius 8d strain was also studied in comparison with co-trimoxazole antibiotic in white mice, which were subcutaneously infected with B. melitensis 520 (Gavrilova et al., 2006). The best result was achieved with using a ten-day cotreatment with a probiotic and antibiotic starting 20 days after infection. After treatment with a probiotic within 20 days after infection, as well as ten-day treatment only with antibiotic starting 20 days after infection, the bacterial load in internal organs was almost at the same level (Gavrilova et al., 2006).
Here, we select a mixture of Lactobacillales; L. plantarum 14d/87 + L. brevis B-3/43 + L. plantarum 14d/19 which showed antagonistic activity against various types of Brucella and pathogens of enteric and concurrent infections of The current study aims to determine the effect of this mixture on the prevention and comprehensive treatment of brucellosis.
MATERIALS AND METHODS
All experiments were conducted in the laboratory of microbiology of the Biological Safety Research Institute. The association A-2, which includes lactobacilli strains; L. plantarum 14d/19 + L. brevis B-3/43 + L. plantarum 14d/87 and L. salivarius 8d strain, was used to evaluate its efficacy against brucellosis. Lactobacillales were cultivated on MRS medium (temperature 35-37°C) for 24 hours. The Lactobacillales antagonism against Brucella was determined using the disk sensitivity method (Anikeev and Lukomskaya, 1977) for three Brucella species (Brucella melitensis, Brucella abortus and Brucella suis), which were cultivated on the Brucella Agar Base medium (prepared according to the protocol (Alton and Jones, 1967)). After keeping the specimens in a thermostat at 37°C for 18-24 hours, the sizes of the zones of lack of Brucella growth around the disks were determined and converted to mm at maximum dilution.
To determine the infection index and the bacterial load in the internal organs of outbred nonlinear white mice, samples were taken from each of them and inoculated on the Brucella Agar Base medium. Infection index was calculated as the proportion (in percent) of infected organs of the total number of examined organs, and the bacterial load was determined as the isolation rate percentage of microorganisms from them (Shumilov et al., 2008).
Statistical analysis of the results was carried out using standard methods of finding the mean values and mean error (Urbakh, 1975).
RESULTS and DISCUSSION
The parameters of the antagonistic activity of bacterial association A-2 and L. salivarius 8d strain against three Brucella strains in comparison with the rifampicin antibiotic are presented in Table 1 and Figure 1.
The association A-2 inhibited the growth of all three Brucella strains at a dilution of 1:10,000 with lysis zone sizes from 15 to 25 mm. L. salivarius 8d activity against B. suis was detected only in the native culture without dilution (15 mm), activity against B. Melitensis was detected at a dilution of 1:10 (17 mm), and activity against B. abortus was detected at a dilution of 1:100 (17 mm) (Table 1, Figure 1). Disks impregnated with rifampicin (5 μg/disk) formed lysis zones (d = 20 mm) for all three Brucella strains. The results show a higher antagonistic activity of the association compared to L. salivarius 8d activity.
Table 1: The antagonistic activity of the association A-2 and L. salivarius 8d strain against Brucella.
| Experiments | Diameter (mm) of Brucella growth inhibition zone by Lactobacillales in dilutions | Control | ||||
| Initial | 1:10 | 1:100 | 1:1,000 | 1:10,000 | Rifampicin | |
| B .melitensis | ||||||
|
L. salivarius 8d |
15±1.0 | 17±1.0 | 0 | 0 | 0 | 20±1.2 |
|
А-2 |
20±1.0 | 22±1.0 | 25±1.1 | 25±1,2 | 25±1.1 | 20±1.0 |
| B. abortus | ||||||
|
L. salivarius 8d |
18±1.0 | 17±1.2 | 17±1.2 | 0 | 0 | 20±1.1 |
|
А-2 |
20±1.1 | 22±1.0 | 25±1.2 | 25±1.1 | 25±1.0 | 20±1.2 |
| B. suis | ||||||
|
L. salivarius 8d |
15±1.3 | 0 | 0 | 0 | 0 | 20±1.1 |
|
А-2 |
16±1.0 | 16±1.2 | 16±1.0 | 15±1.2 | 15±1.1 | 20±1.2 |
Table 2: Seed of internal organs of experimental mice.
| Bacterial load of internal organs, % | |||||||
| lymph nodes | Organs | ||||||
| postpharyngeal | lower cervical | inguinal | para-aortal | Liver | kidneys | spleen | heart |
| First group (probiotic for five days before infection) | |||||||
| 50±1.5 | 70±1.8 | 50±1.4 | 40±1.3 | 50±1.6 | 50±1.4 | 70±2.0 | 20±1.1 |
| Second group (probiotic for therapeutic purposes for 20 days after infection) | |||||||
| 60±1.3 | 0 | 10±0.2 | 10±1=0.1 | 30±0.7 | 10±0.2 | 70±1.8 | 10±0.1 |
| Third group (probiotic in combination with the antibiotic starting 20 days after infection) | |||||||
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fourth group (antibiotic starting 20 days after infection) | |||||||
| 0 | 0 | 0 | 0 | 0 | 0 | 10±0.2 | 0 |
| Fifth group (control) – 20 days after infection | |||||||
| 100±00 | 100±1.0 | 80±1.6 | 80±1.5 | 80±1.0 | 50±0.9 | 100±0.9 | 30±0.4 |
| Sixth group (control) – 30 days after infection | |||||||
| 90±0.9 | 90±0.7 | 100±0.0 | 100±0.9 | 100±1.0 | 50±0.8 | 100±1.0 | 40±0.4 |
The animals experiment was conducted on white mice, six groups (ten mice in each group) were used after keeping them in quarantine for five days. Mice were fed a balanced diet. Experimental animals received medications orally 15-20 minutes before feeding. The first group received the probiotic orally for five days before infection at a 4% dosage in addition to the daily feed intake. The second group received the probiotic for 20 days after infection at a 7% dosage in addition to the daily feed intake. The third group received the probiotic for ten days (starting 20 days after infection) at a 7% dosage in addition to the daily feed intake together with the rifampicin antibiotic, which was administered orally at a dose of 5.0 mg per mouse, dissolved in 0.5 ml of sterile distilled water. The fourth group received only the rifampicin antibiotic orally at a dose of 5.0 mg per mouse, dissolved in 0.5 ml of sterile distilled water. The fifth and sixth groups (control groups) were not treated with drugs before and after infection.
All groups were infected with a 48-hour virulent culture of Brucella melitensis 16M strain at a titrated dose of 105 CFU in 0.5 ml of saline subcutaneously in the inguinal region. The Brucella melitensis 16M strain was previously passaged for five times through the body of sensitive guinea pigs. After the fifth passage, a 48-hour culture of B. melitensis 16M strain in doses of 5, 10, and 20 microbial bodies (mb) was used to infect animals and cause a generalized form of brucellosis.
The autopsy of animals with bacteriological studies of eight internal organs for the first, second, and fifth groups was carried out after the following periods after 20 days while the third, fourth, and sixth groups was after 30 days. Bacteriological inoculation of pathological material from animals was carried out on a dense and liquid Brucella broth base medium from lymph nodes (postpharyngeal, lower cervical, inguinal, and para-aortal) and organs (liver, kidneys, spleen, and heart). The inoculum was placed in a thermostat at a temperature of +37°C for 30 days.
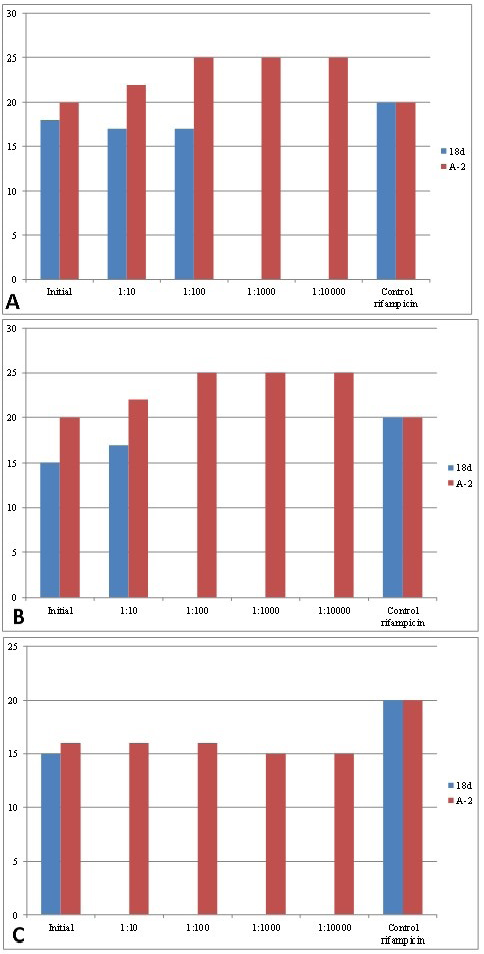
Figure 1: Antagonistic activity of the created association of lactic acid bacteria and L. salivarius 8d strain against brucellae. Note: (a) B. abortus; (b) B. melitensis; (c) B. suis.
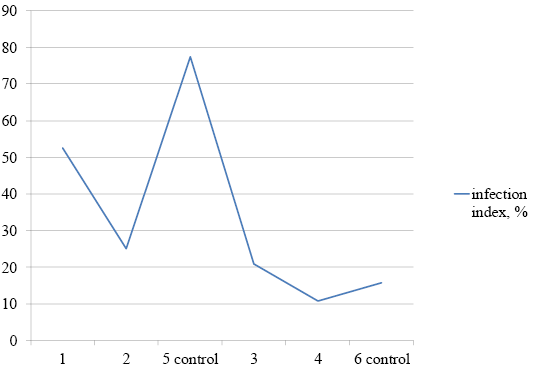
The first growth properties of the original Brucella melitensis 16M strain were observed three to four days after inoculation. Our results showed that the infection index was 52.5% in animals of the first group (that received the probiotic for five days before infection), 25% in the second group (that received the probiotic for therapeutic purposes for 20 days after infection), and 77.5% in the control group. Consequently, the infection of internal organs decreased by 25% in the first group of animals and by 58.75% in the second group. In the third group of animals (that received the probiotic in combination with the antibiotic starting 20 days after infection), no infected internal organs were found. In the control group, the infection index was 83.75%. In the fourth group of animals (that received the antibiotic), the infection index was 1.3% (Figure 2).
In all experimental groups of animals, the intensity of contamination of the internal organs also decreased compared with the control (Table 2).
Compared to the control, in the first group, the bacterial load was 50% lower in the pharyngeal lymph nodes, 40% lower in the para-aortal lymph nodes, 30% lower in the liver, spleen, and lower cervical and inguinal lymph nodes, and 10% lower in the heart. The lower cervical lymph nodes and spleen had the highest bacterial loads (70%); in control animals, this indicator for these organs was 100%. In the second group, the bacterial load was 90% lower in the inguinal and paraortral lymph nodes, 40% lower in the kidneys, 70% lower in the liver, and 30% lower in the pharyngeal lymph node, heart, and spleen. Brucella bacterial load was not detected in the lower cervical lymph nodes; this parameter in the control was 90%. No bacterial load was detected in the internal organs of animals in the third group; it ranged from 40 to 100% in the control. In the fourth group, Brucella bacterial load was detected only in one case in the spleen (10%).
CONCLUSION
Therefore, the usage of probiotics for prophylactic and therapeutic purposes significantly reduce the infection index and the bacterial load in the internal organs of беспородной нелинейной white mice infected with a highly virulent B. melitensis 16M strain.
So, the usage of the new probiotics will be an important strategy for the prevention of brucellosis in farm animals, as well as the preservation and restoration of public health leading to reduced losses as a result of forced slaughter of animals with brucellosis and the cost of treatment.
Authors Contribution
All authors contributed equally.
Conflict of interest
The authors have declared no conflict of interest.
REFERENCES