Advances in Animal and Veterinary Sciences
Research Article
Assessment of Serum Mineral Concentrations of Barki Sheep and its Impact on Kidney Functions in El-Hammam City
Marwa M. Morsy1*, Abd El-Rehim A. El-Ghannam2, Sherif Y. Saleh2, Mahmoud M. Arafa3
1Department of Biochemistry, Animal Health Research Institute, Matrouh Lab, Agricultural Research Center, Egypt; 2Department of Biochemistry, Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt; 3Department of Biochemistry, Toxicology, and Deficiency diseases, Animal Health Research Institute, Agricultural Research Center, Dokki, Egypt.
Abstract | Minerals are essential for the health and productivity of livestock. The present study was conducted to determine the serum concentrations of lead (Pb), cadmium (Cd), copper (Cu), zinc (Zn), iron (Fe), and selenium (Se) of Barki sheep reared in El-Hammam city, Egypt. Clinically healthy Barki sheep (n=40, 2-4 years) were randomly selected in equal numbers from two different farms. Another twenty healthy Barki sheep (2-4 years) were obtained from another farm as control. Animals from three different farms were classified according to water source: Borg El Arab as control (Tap water); El-Hammam I (Surface water); El Hammam II (groundwater). Water and serum samples were prepared for the detection and estimation of selected minerals using Flame Atomic Absorption Spectrometer. Serum BUN and creatinine concentration were also assessed as renal function parameters. The results revealed an alteration in metals concentration in water samples from one site to another. A higher Pb and Se levels were El Hammam II (GW, 1.21ppm) followed by El Hammam I (SW, 0.88ppm) and control (TW, 0.83 ppm).The levels of Cu, Zn, and Fe were below EWQS limits, while Cd was not detected in all samples. For serum metal analysis, variable concentrations were detected in examined sheep of different groups. Serum concentration for Fe and Se were significantly higher (P<0.001), while it was reduced considerably (P<0.001) for Cu and Zn. However, the serum levels of heavy metals, including Pb and Cd, were low to be detected. The results of BUN were significantly elevated (P<0.001) in examined sheep of El-Hammam city than the control, while no significant changes in creatinine levels (P>0.05) were observed between groups. It is concluded that groundwater quality needs improvement and continuous monitoring for water quality. The animal needs to be fed with oral supplementation of required minerals, and further studies in subject matter are necessary to ascertain study outcome and its practical applications further.
Keywords | Barki sheep, Heavy metal, Trace element, Kidney Function tests
Received | June 10, 2020; Accepted | June 25, 2020; Published | July 10, 2020
*Correspondence | Marwa M. Morsy, Department of Biochemistry, Animal Health Research Institute, Matrouh Lab, Agricultural Research Center, Egypt; Email: dr1m@live.com
Citation | Morsy MM, El-Ghannam AA, Saleh SY, Arafa MM (2020). Assessment of serum mineral concentrations of barki sheep and its impact on kidney functions in El-Hammam city. Adv. Anim. Vet. Sci. 8(s1): 68-75.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.s1.68.75
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Morsy et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Sheep, the most abundant ruminant livestock species in Egypt (FAO, 2014), is used mainly as a source of meat, and wool serves as a secondary product (Elshazly and Youngs, 2019). The current number of sheep in Egypt is 5.69 million heads (FAO, 2017). The one-third population is kept in the western desert, especially in the north-western coastal area. Barki sheep, the dominant coarse wool fat-tailed breed of this area, is known to be well-adapted to harsh climates, reduced feeding, and diseases (Tibbo et al., 2008). Indeed, small ruminant production constitute a major part of the Bedouin’s income. The native rangelands are deteriorating due to environment- and human-associated interventions (Halmy, 2019). For instance, from 1995 to 2010, with an annual rainfall of less than 150 mm, sheep production in Matrouh governorate has declined sharply due to severe 15-year drought in the north-western coastal zone (Osman et al., 2012; Alary et al., 2014).
Minerals are essential to support the health and productivity of animals. Numerous studies have revealed that a mineral deficiency or even imbalance can lead to various disorders, impaired growth, reduced reproduction, and depressed immunity of livestock (Gonul et al., 2009; Dar et al., 2014; Elayaraman et al., 2019). Among others, trace minerals, the copper, zinc, iron, and selenium, are required for normal biochemical processes in the body. Being a part of several enzymes, such as superoxide dismutase, glutathione peroxidize, and glutathione reductase (Evans and Halliwell, 2001), they are considered essential to maintaining animal health and productivity.
Several studies reported an effect of metal toxicity on kidney functions (Bazargani-Gilani et al., 2016; Fevrier-Paul et al., 2018; Madrigal et al., 2019). The organ is recognized as one of the most common targets for the toxicity of the drug and environmental chemicals (Khan et al., 2013). The organ also serves as the filtration apparatus of blood, where it facilitates the excretion of toxins and by-product of metabolic processes, especially the nitrogenous compounds such as urea and creatinine (Gounden and Jialal, 2018).
The evaluation of the mineral status of livestock is an essential tool in health management. Adequate provision of essential trace elements is necessary to avoid nutritional disturbance and to sustain animal production efficiency and welfare (FAO, 2012). With little or no mineral supplements, Barki sheep are mainly maintained on grazing in El Hammam city. Since mineral deficiency has often been associated with wool loss and lameness (Hill and Shannon, 2019), the study was conducted to investigate the serum mineral content of blood sheep and water sources in El Hammam city to devise a strategy for optimum production performance and prevention of health disorders.
Materials and Methods
Ethical approval
The study was performed according to the guidelines of the Animal Health Research Institute. All procedures were approved by the scientific research committee, Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt.
Study area
El-Hammam city (30° 50′ 30.67″ N, 29° 23′ 38.56″ E) is located in the north-western coastal zone of Egypt (Figure 1). The city elevation range from 0 to more than 40 meters above sea level and slopes towards the Mediterranean Sea. The climate is typically arid to semi-arid and is characterized by long, hot, and dry summer, mild winter with little rainfall, and moderate to high relative humidity (Bornkamm and Kehl, 1989). The study area consisted of the irrigated and groundwater region. Originating from the river Nile, the Sheikh Zayed canal extends from the El Hammam canal to irrigate the region. The groundwater region depends upon coastal aquifer, which mostly contains brackish water recharged annually by local rainfall and the Nile seepage water from El-Nasr, El-Hammam, and Maryut canals (Sayed, 2013). Without the supplementation of either concentrates or minerals, the Barki flocks are restricted to limited areas for grazing pastures grown around surface water canals and groundwater wells.
Animals and study design
During the spring season of 2018, forty Barki sheep (2-4 years) of both sexes were selected randomly from two different flocks in El Hammam city at Matrouh governorate. According to the source of water, animals were grouped as El-Hammam I (Surface water) and El Hammam II (groundwater).The randomly selected sheep were neither pregnant nor had parturition recently. All examined study-included animals were clinically healthy. Sheep were fed via grazing and reared under similar management conditions. Another twenty healthy Barki sheep (2-4 years) were obtained from Animal Production Research Station at Borg El Arab and considered as control (Tap water). Animals were clinically healthy, nonpregnant, free from parasites, and vaccinated against infectious disease. They were offered ordinary ration and kept under complete veterinary supervision.
Sampling
All glass tubes and plastic containers were cleaned twice with diluted HNO3, rinsed with deionized distilled water twice and air-dried before use.
Water samples collection
A 250 ml of a water sample from each ranch was collected in acid leached polyethylene bottles according to the recommendation of the American Public Health Association (APHA, 2012).
Blood sample collection
Five milliliters of blood was collected from the jugular vein of each of the sheep into a labeled centrifuge tube without anticoagulant. Serum was separated by centrifugation of at 3000 rpm for 20 minutes and stored at -20°C until further analysis.
Mineral analysis
Water samples were prepared according to the method described by AOAC (2016). Briefly, the samples were filtered through a cellulose membrane (0.45 µm, Millipore), digested in 3 ml HNO3, then heated and evaporated for dryness. The sample was cooled, added with 3 ml HNO3, and heated again to complete digestion. To dissolve residue, 2 ml HCl was added and heated gently. The final solution obtained from the digestion procedure was diluted with deionized water.
Serum samples were prepared following the Meret and Henkin (1971) protocol where serum sample was diluted (1:10) with a 6% solution of 2-butanol.
Water and serum samples were analyzed by Flame Atomic Absorption Spectrometer (model SensAA, GBC, Australia) (Meret and Henkin, 1971; AOAC, 2016) in Animal Health Research Institute. The Pb, Cd, Cu, Zn, Fe, and Se concentrations were detected in each of the samples at suitable wavelengths of 217.0, 328.1, 324.7, 213.9, 248.3, and 196.0 nm, respectively. For the estimation of specific metals according to the manufacturer’s recommendations, other instrumental parameters such as bandwidth and lamp current were set-up separately. Highly purified deionized water was used to prepare to calibrate standards. Appropriate quality assurance procedures and precautions were considered to ensure the reliability of the results. The results of the water samples were compared with the MPL of Egyptian drinking water quality standards (EWQS, 2005).
Serum BUN and creatinine
The level of blood urea nitrogen (BUN) and serum creatinine was determined colorimetrically as per the method described by Vassault et al. (1986) and Young et al. (1975). The kits were obtained from Biodiagnostic Company, Egypt.
Statistical analysis
All data were expressed as mean ± standard error, and the variance was analyzed by one-way analysis of variance (ANOVA) followed by Duncan’s multiple range test (DMRT) using SPSS Version 25. Differences were considered significant at p<0.05.
Results
The concentrations of selected heavy metals (Pb, Cd) and trace elements (Cu, Zn, Fe, Se) in the collected water and serum samples are given in Table 1 and Table 2, respectively.
Table 1: Concentrations (ppm) of selected minerals (Pb, Cd, Cu, Zn, Fe, Se) in different sources of water in El-Hammam city compared to Egyptian drinking water standards.
|
Minerals
(ppm)
|
Locality | EWQS | ||
| Control (TW) | El Hammam I (SW) | El Hammam II (GW) | ||
| Pb | 0.07 | 0.11 | 0.19 | 0.01 |
| Cd | ND | ND | ND | 0.003 |
| Cu | 0.053 | 0.053 | 0.021 | 2 |
| Zn | 0.044 | 0.104 | 0.09 | 3 |
| Fe | 0.039 | 0.08 | 0.065 | 0.3 |
| Se | 0.83 | 0.88 |
1.21 |
0.01 |
ND: Not detectable; TW: tap water; SW: surface water; GW: groundwater. EWQS (Egyptian drinking water quality standards, Decision number 1589/2005).
Mineral concentrations in water samples
Mineral concentrations of water samples (tap water, surface water, and groundwater) in comparison with Egyptian drinking water standards limits (EWQS, 2005) are given in Table 1. The Pb and Se levels in all water samples were recorded above MPL. There is no clear difference between areas in Pb concentration, but a higher concentration was recorded in El Hammam II (GW, 0.19ppm), El Hammam I (SW, 0.11ppm), and control (TW, 0.07 ppm). Similarly, the concentrations of selenium were recorded above the EWQS limit (0.01 ppm) in El Hammam II (GW, 1.21ppm) followed by El Hammam I (SW, 0.88ppm) and control (TW, 0.83 ppm).However, the levels of Cu, Zn, and Fe were below the EWQS limits as 2, 3, and 0.3 ppm, respectively. Furthermore, in all the collected water samples, the concentrations of Cd were low to be detected.
Mineral concentrations in serum samples
Mean concentrations and ranges of selected mineral concentrations in serum of Barki sheep are given in Table 2. The Pb and Cd levels were too low to be detected in all serum samples of different groups. Conversely, there were significant differences for concentrations of Cu, Zn, Fe, and Se among the examined groups. The mean serum concentration of Cu was significantly decreased (P < 0.001) in examined sheep compared to the control. The concentration of Cu was found to be 1.72 ± 0.06 ppm for the control, 1.03 ± 0.07 ppm El Hammam I (SW), and 0.45 ± 0.06 ppm El Hammam II (GW). The mean Zn concentration in EL Hammam I (SW) was found to be within an adequate range (0.69±0.06 ppm). However, compared to the control, it was found to have a significant difference (P < 0.01) for animals originating from EL Hammam II (GW), where its concentration was 0.38±0.06 ppm. The mean serum Fe concentration of the El Hammam I (SW) group () was higher than the control (3.32 ± 0.74 ppm v/s 2.12 ± 0.07 ppm); however it was lower in El Hammam II (GW) group (1.13 ± 0.16 ppm). The mean serum Se concentration was significantly (P < 0.001) higher in sheep of El Hammam I (SW) (4.28±0.65 ppm) followed by EL Hammam II (GW) (1.57±0.22 ppm), and the control (0.18 ± 0.04 ppm).
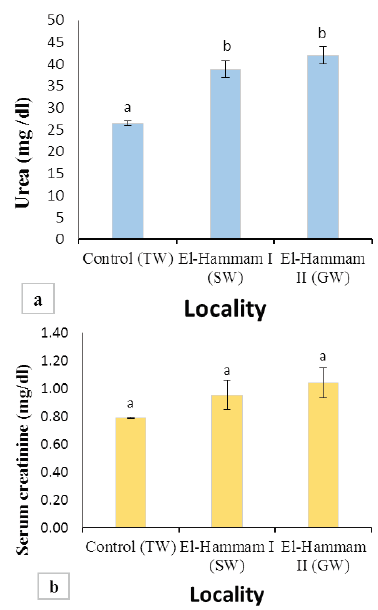
Figure 2: (a) Values of BUN in Barki sheep from surveyed localities in El-Hammam city. (b) Values of serum creatinine in Barki sheep from surveyed localities in El-Hammam city. (Each bar represents the mean ± SE with Superscript a refers to a significance from control group (P< 0.05).
Serum BUN and creatinine
Results of BUN and serum creatinine of different groups are presented in Figure 2. The mean value of BUN was significantly increased (P<0.001) in sheep of El Hammam (38.76±1.91 and 42.00±1.98 mg/dl) than control (26.47±0.56 mg/dl) (Figure 2a). However, the mean value of serum creatinine levels in all groups was not different significantly (P>0.05). It was found to be 0.79±0.01, 0.96±0.11, 1.04±0.11 mg/dl for the control group, EL Hammam I (SW), and EL Hammam II (GW) groups, respectively (Figure 2b).
Discussion
Drinking water quality is a worldwide concern. Therefore, it is important to monitor its quality and evaluate the risk of exposure to heavy metals (Priti and Paul, 2016). The obtained concentrations of six elements analyzed in different sources of water samples are summarized in Table 1. The Pb concentrations in all water samples from different areas were more than the permissible limit (0.01 ppm) according to Egyptian drinking water quality standards (EWQS, 2005). These findings are in agreement with the various studies conducted previously (El Gohary et al., 2017; El-Baz and Khalil, 2018). The Cd concentration was not detected in all water samples. It is considered one of the most toxic metals in the environment with a wide range of organ toxicity and long elimination half-life (Patrick, 2003). Different sources of Cd, such as industrial activities or intensive agricultural practices, contribute to its environmental contamination. The concentrations of Cu, Zn, and Fe were below Egyptian drinking water quality standards (EWQS, 2005). The concentration of Se in the study area showed a wide range of variation. The highest concentration of Se was found in El Hammam II (Groundwater, 1.21 ppm) followed by El Hammam I (Surface water, 0.88 ppm) and control (Tap water, 0.83 ppm), respectively. These recorded values were higher than the permissible limit (0.01 ppm) EWQS (2005). Due to higher contact times of rock-water interactions, selenium concentration in groundwater is higher than surface water (Fordyce, 2013). Recently, high levels of selenium are steadily found in groundwater and drinking water around the world (Golubkina et al., 2018).
Lead and Cadmium are toxic elements that represent an indicator of environmental pollution. In the present study, Lead and Cadmium concentrations in serum of Barki sheep were not detected from different surveyed localities (Table 2). These findings are compatible with the water analysis of both regions of El-Hammam city. Tuncer (2019) found that Pb and Cd levels in the sheep lie below the acceptable limits. Therefore pastures could be considered safe in terms of food safety and environmental pollution with heavy metals. The mineral concentration of Cu, Zn, Fe, and Se in
Table 2: Statistical summary of concentrations (ppm) of selected minerals (Pb, Cd, Cu, Zn, Fe, Se) in blood serum of Barki sheep in El-Hammam city, along with reference values.
|
Minerals
(ppm)
|
Locality | P-value | Ref. value | |||||
| Control (TW) | El Hammam I (SW) | El Hammam II (GW) | ||||||
| Range | Mean±SE | Range | Mean±SE | Range | Mean±SE | |||
| Pb | ND | ND | ND | ND | ND | ND | – | – |
| Cd | ND | ND | ND | ND | ND | ND | – | – |
| Cu | 1.55–1.90 |
1.72c±0.06 |
0.80–1.17 |
1.03b±0.07 |
0.31 – 0.64 |
0.45a±0.06 |
<0.001 | 0.75 - 1.7 |
| Zn | 0.55–0.86 |
0.70b±0.05 |
0.51–0.87 |
0.69b±0.06 |
0.21 – 0.54 |
0.38a±0.06 |
0.003 | 0.55 - 1.2 |
| Fe | 1.99–2.32 |
2.15ab±0.06 |
1–5.09 |
3.32b±0.74 |
0.7 – 1.55 |
1.13a±0.16 |
0.014 | 0.9 - 2.7 |
| Se | 0.09 – 0.3 |
0.18a ±0.04 |
2.25–6.05 |
4.28c±0.65 |
ND – 2.08 |
1.57b±0.22 |
<0.001 | 0.06 - 0.2 |
Means of different variables within the same raw having different superscripts are significantly different (P < 0.05). ND= Not detectable. Reference values according to Herdt and Hoff (2011).
the sera of the control group were within reference ranges for domestic sheep (Herdt and Hoff, 2011) (Table 2).
Copper is an essential trace element that presents a variety of functions in animal organisms. It plays a key role in enzyme function, maturation of red blood cell, collagen synthesis, and immune response (Suttle, 2010). Mean serum concentrations of Cu were significantly reduced (P<0.001) in El-hammam city groups than control (Table 2), suggesting that its values range from deficient to marginal levels. Secondary deficiencies of Cu could be due to interference with other minerals provided in excess, such as Zn, Fe, S, or Mo in the diet (Underwood and Suttle, 1999). There are a variety of physiological and biochemical changes associated with copper deficiency such as reduced appetite, anemia, achromotrichia (lack of pigmentation of colored wool), a reduced growth, an impairment of immune function, and consequently an increased susceptibility to disease (Ebrahim, 2015; Hefnawy et al., 2017).
Zinc is an essential trace element for animals. It is involved in protein synthesis and is a constituent of many metalloenzymes (Underwood and Suttle, 1999). Based on our findings, we observed a non-significant difference between the mean serum Zn concentrations of the El hammam (SW) group and the control. On the other hand, the mean serum Zn concentrations of the El hammam (GW) group were 0.38 ± 0.06 ppm (range: 0.21–0.54 ppm), which is significantly lower (P<0.01) than the value observed in control (0.70 ± 0.05 ppm). These observations correspond to (Song and Shen, 2019), who found zinc deficiency in the Wumeng semi-fine wool sheep mainly due to the low content of zinc in soil and forage. While inducing experimental Zn deficiency, Ibrahim et al. (2016) noted a negative impact on the general health condition, some hematological and immunological parameters in sheep. has. Sera Zn concentrations are highly variable and depend on several variables, including dietary Zn intake, infection, inflammation, stress, and injury (Kincaid and Hodgson, 1989). Iron is an essential element for humans and animals. The main portion of Fe is bound to hemoglobin. Fe is also essential in the prosthetic groups of numerous enzymes and regulatory proteins (NRC, 2007; Kohgo et al., 2008; Zhang, 2014). The mean values of Fe in the serum of Barki sheep from the El Hammam (SW) group were significantly higher than the control. The Fe toxicity is rarely experienced in ruminants because of the absorption limit when levels are high in the diet (Herdt and Hoff, 2011). Mean serum Fe concentration in El Hammam (GW) group was significantly lower than the control group; however, were within the reference range recommended (Herdt and Hoff, 2011). Forage showed marked seasonality in Fe concentrations where its peak occurs in spring and autumn. Though most livestock feeds contain a high concentration of Fe, a few cases of deficiencies are documented (Suttle, 2010).
Selenium is a component of several selenoproteins and selenoenzymes with vital biological functions such as antioxidant activity, anti-inflammatory, antagonistic and anti-carcinogenic effects (Hosnedlova et al., 2017). In the current study, mean serum selenium concentrations reported from two different flocks in EL Hammam I (SW) and II (GW) groups were 4.28±0.65 ppm (range: 2.25–6.05ppm) and 1.57±0.22 ppm (range: ND–2.08ppm), respectively. Such high selenium levels of Barki sheep from the El hammam (SW) group indicate that they had been exposed to elevated levels of selenium. These values were significantly higher (p<0.001) than the control indicating an individual variability to selenium tolerance in sheep. Similar observations are reported previously by several researchers (Pavlata et al., 2011; Hall et al., 2012; Faixová et al., 2016; Saeed et al., 2019). Cristaldi et al. (2005) reported a significant elevation of serum Se levels (P < 0.001) after supplementation in the diet. Indeed, ruminants are better adapted to a diet containing excessive selenium than mono-gastric simply because the ruminal microbiota metabolizes selenium into a less absorbable form and gets excreted in the feces (Colegate and Dorling, 1994). Serum levels of blood urea and creatinine are common markers of kidney function (Ferguson and Waikar, 2012). Figure 2 revealed a significant increase (P<0.01) in the blood urea level of EL Hammam (SW and GW) groups than the control. Though a considerable elevation in serum creatinine levels of EL Hammam (SW and GW) groups was observed as compared to the control group, the difference was not significant. Similar to these observations, Juniper et al. (2006), Slavik et al. (2008), and Khalifa et al. (2016) reported a significant increase in blood urea levels when cows were fed with inorganic Se supplementation (sodium selenite). Conversely, Biazus et al. (2018) found no significant effect on blood urea nitrogen upon supplementation with diphenyl diselenide in dairy sheep. These contradicting results correspond to dietary degradable protein, which may lead to a significant increase in BUN concentration in urine (Canfield et al., 1990; Hassan and Saeed, 2012) because urea is a good indicator of the dietary protein intake (Schröder et al., 2003).
Conclusions
These results provide a database for the mineral status of Barki sheep and their drinking water quality in El Hammam city. Cd, Cu, Zn, and Fe concentrations in water were within the recommended levels by EWQS; however, it was not true for Pb and Se and therefore needed necessary interventions. Serum analysis revealed deficient to marginal levels of Zn and Cu in sheep of El Hammam city. These results could be used to improve the animal diet and subsequent rate of growth and reproduction.
Acknowledgments
The first author is thankful to the Director, Dr. Sohair Rashad, Animal Health Research Institute, Matrouh lab, Egypt, for providing facilities to accomplish this work. Special thanks are also to Dr. Mohamed Saed, Assistant Researcher, for assistance during the sample collection period.
Authors Contribution
MMM conceived the study, carried out its design and manuscript drafting. MMA helped in the laboratory work and revised the paper. AAE and SYS supervised the research. All authors read and approved the final manuscript.
Conflict of interest
The authors have declared no conflict of interest.
References