Advances in Animal and Veterinary Sciences
Research Article
Prevalence of Escherichia coli in Marked Poultry Carcasses in Egypt
Esraa A. Abdelkarim1*, Abd-Elsalam E. Hafez1, Mohamed A. Hussein1, Tamer S. Elsamahy2
1Food Control Department, Faculty of Veterinary Medicine, Zagazig University, Zagazig 44519, Egypt; 2Biofuels Institute, School of the Environment and Safety Engineering, Jiangsu University, Zhenjiang, 212013, China.
Abstract | This study evaluated the incidence of E. coli in retail chicken meat in El-Sharkia- Egypt, and the effectiveness of zinc peroxide nanoparticles (ZnO2-NPs) against pan drug-resistant bacteria (PDR) isolated from chicken meat. A total of 403 chicken meat cuts-up samples were collected. The presence of E. coli was tested by the growth on selective medium and biochemical identification followed by antibiogram assay. Various virulence factors were phenogenotypically analyzed. The data revealed a 66.3% incidence of E. coli. The incidence in the fresh cuts-up sample was significantly higher than other samples (chilled and frozen). The incidence of multidrug-resistant (MDR) strains was found to be 81%. Genotypic characterization revealed the absence of stx1 from all strains, while the stx2 gene was found in 29% of PDR isolates. Also, ZnO2-NPs showed an efficiency against all PDR E. coli strains.
Keywords | Foodborne diseases, E. coli, Antimicrobial resistance genes, Antibiotics, Zinc peroxide nanoparticles
Received | June 10, 2020; Accepted | June 25, 2020; Published | July 10, 2020
*Correspondence | Esraa A. Abdelkarim, Food Control Department, Faculty of Veterinary Medicine, Zagazig University, Zagazig 44519, Egypt; Email: esraaabdelkrim92@gmail.com
Citation | Abdelkarim EA, Hafez A-EE, Hussein MA, Ali SS, Elsamahy TS (2020). Prevalence of escherichia coli in marked poultry carcasses in Egypt. Adv. Anim. Vet. Sci. 8(s1): 55-61.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.s1.55.61
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Abdelkarim et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Escherichia coli (E. coli), Staphylococus aureus (S. aureus), Pseudomonas aeruginosa (P. aeruginosa), and Salmonella spp. have caused significant health-associated problems for human (Saltoglu et al., 2018). Extensive and frequent use of antimicrobial chemicals is further aggravating the situation. To overcome this condition, it is necessary to develop new anti-bacterial agents (Ali et al., 2019; Ali et al., 2020).
The meat of chicken carcasses can be contaminated by foodborne bacteria such as Campylobacter jejuni, Salmonella spp., Clostridium perfringens, Staphylococcus aureus, and E. coli during the processing operation such as scalding, de-feathering, evisceration, processing equipment and contamination from other birds (Morshdy et al., 2018). Among these, E. coli is believed to be the most critical pathogens worldwide, particularly Egypt (Osman et al., 2015). The pathogen is responsible for several infectious diseases such as tissue and skin infections, osteomyelitis, pneumonia, sepsis, mastitis, arthritis, and endocarditis (Tong et al., 2015). The clinical significance of E. coli is due to its ability to acquire antibiotic resistance rapidly and secrete virulence factors. In this regard, Shiga toxin-producing E. coli have super antigenic activity, and almost half of the known E. coli strains are known to cause emesis. Therefore, this pathogen is considered a potential hazard for consumers (Hennekinne et al., 2012).
Zinc peroxide nanoparticles (ZnO2-NPs) are deemed safe for humans and animals. With perfect chemical stability, biocompatibility, and low toxicity, the ZnO-NPs are thought to have antibacterial and antifungal properties (Samanta, 2017). To our knowledge, this is the first report to assess the prevalence and incidence of E. coli in retail chicken meat in El-Sharkia Governorate and estimate the activity of synthesized ZnO2-NPs against drug-resistant E. coli strains.
MATERIALS AND METHODS
Collection of samples
A total of 403 marketed chicken meat samples, including breast, thigh, lung, liver, and gizzard cuts-up, were purchased from different local markets in El-Sharkia, Egypt. The samples were transported to the laboratory in a sterilized bag to avoid cross-contamination. A 25 gram of different parts of each sample was separately grounded in a sterile mechanical blender, mixed with 225 ml of sterile buffered 0.1 % peptone water, and mixed in a stomacher for 2 min (Seward, BA6021, UK) (ICMSF, 1986). One ml of the homogenate was used to prepare tenfold serial dilution (10-4-10-8).
Bacteriological examination
One ml from each dilution was inoculated in MacConkey broth and incubated at 44°C for 24-48 h. After that, the inoculum was streaked onto eosin methylene blue (EMB) agar plates and incubated for 24 h at 37°C. The selected isolates were subculture on blood agar plates (BAP) to detect the positive isolates for β-hemolysis and these positive isolates were subjected to l-pyrrolidonyl-β-naphthylamide (PYR) test based on the identification scheme of York et al. (2000). The selected isolates were subjected to bacterial cell motility, and the biochemical analyses, including Methyl Red/Voges-Proskauer (MR/VP), hydrogen sulfide production, indole production, oxidase, catalase, urease, citrate, and gelatinase reaction as per the method described previously (Holt et al., 2000). The presumptive E. coli colonies were selected based on phenotypic characterization and subcultured on tryptic soy agar (TSA) medium to obtain pure bacterial cultures.
Antimicrobial sensitivity testing
Drug sensitivity was evaluated by the disc diffusion method. The bacterial culture was inoculated onto Muller-Hinton agar along with 11 antibiotic discs containing; gentamicin (CN), imipenem (IPM), cephalothin (KF), ciprofloxacin (CIP), vancomycin (VA), colistin sulfate (CT), ampicillin (AMP), chloramphenicol (C), trimethoprim/sulfamethoxazole (SXT), tetracycline (TE) and amoxicillin/clavulanic acid (AMC). The results were interpreted as resistant, sensitive, or intermediate depending on the Clinical and Laboratory Standards Institute (CLSI) guidelines (CLSI, 2017) as shown in Table 1. MDR was defined as resistance to at least three or more classes of antimicrobial agents. However, pan drug-resistant (PDR) was defined as non-susceptibility to all agents in all antimicrobial categories (Ali et al., 2016; El-Shouny et al., 2016). The multiple antibiotic resistance index (MAR) was also calculated as previously reported by Morsy et al. (2017).
Table 1: Antimicrobial agents and zone interpretive chart.
| Antimicrobial group | Antimicrobial agent | Disk content (µg/ml) | Breakpoints | ||
| Susceptible | Intermediate | Resistant | |||
| Aminoglycosides | Gentamicin (CN) | 10 | ≥ 15 | 13-14 | ≤ 12 |
| Carbapenems | Imipenem (IPM) | 10 | ≥ 19 | 16-18 | ≤ 15 |
| Cephalosporin | Cephalothin (KF) | 30 | ≥ 18 | 15-17 | ≤14 |
| Fluoroquinolones | Ciprofloxacin (CIP) | 5 | ≥21 | 16-20 | ≤15 |
| Glycopeptides | Vancomycin (VA) | 30 | ≥17 | 15-16 | ≤14 |
| Lipopeptides | Colistin sulphate (CT) | 10 | ≥11 | - | ≤10 |
| Penicillins | Ampicillin (AMP) | 10 | ≥17 | 14-16 | ≤13 |
| Phenicols | Chloramphenicol (C) | 30 | ≥ 18 | 13-17 | ≤ 12 |
| Sulphonamide | Trimethoprim/sulfamethoxazole (SXT) | 1.25/23.75 | ≥16 | 11-15 | ≤10 |
| Tetracyclines | Tetracycline (TE) | 30 | ≥ 19 | 15-18 | ≤ 14 |
|
β-Lactam |
Amoxycillin/clavulanic acid (AMC) | 20/10 | ≥ 18 | 14-17 | ≤13 |
Phenotypic characterization of virulence factors
In order to perform the haemolysin test, the presumptive E. coli isolates were inoculated onto sheep blood agar (5%) and incubated overnight at 37 ºC. Haemolysin production was detected by the presence of lysis, a zone of complete lysis around the colony.
The gelatinase production was evaluated using gelatine agar. The plate was inoculated with the bacterial suspension and incubated at 37 ºC for 24 h. The plate was then flooded with a mercuric chloride solution. The halo zone around the colonies was considered to be positive for gelatinase.
For mannose resistant fimbrial haemagglutination (MRHA) test, E. coli growing colonies were inoculated into 5 ml of Muller Hinton broth (MHB) to give a turbid suspension of the standard McFarland’s solution (2.4 x 109 CFU/ml) then incubated for 5 days at 37ºC. The pellicle on the surface was noted and it was subcultured onto Colonization Factor Antigen agar (CFA) and incubated overnight at 37 °C. The group A positive venous blood was added to equal amounts of the Alsever’s solution, this was washed three times and an erythrocyte suspension (3%) was made in phosphate-buffered saline (PBS; pH 7.4). A volume of 40 μl of this erythrocyte suspension was added to 40 μl of PBS and 40μl of D-mannose (3%) in a different well of the same VDRL slide and mixed with the colonies from the CFA agar plates in both the wells. The slides were placed on a VDRL rotator for 4 min. and the haemagglutination reactions were recorded after 2, 5, 10, and 15 min. The haemagglutination was considered to be mannose resistant when it occurred in the presence of D-mannose and it was considered to be mannose sensitive when it was inhibited by D- mannose (Shruthi et al., 2012).
Cell surface hydrophobicity (CSH) was detected using salt aggregation tests. E.coli grown isolates were inoculated into 1 ml of phosphate buffer (pH 6.8) and the turbidity was adjusted to Mcfarland’s standard 6 to get a colony count of 5×109 CFU/ml. Different molar concentrations of ammonium sulfate solutions ((NH4)2SO4; 1M, 1.4 M and 2 M) were prepared. A volume of 40 μl of the bacterial suspension was mixed with an equal volume of the (NH4)2SO4 of different molarities on a VDRL slide. The slides were placed on a VDRL rotator for 4 minutes and the clumps which were formed in different concentrations of the (NH4)2SO4 were observed. The E. coli strains were considered as hydrophobic if they aggregated in the (NH4)2SO4 solution of concentration, <1.4M.
Genotypic characterization
Following (Pincus, 2006), serotyping was determined for PDR E. coli isolates using the VITEK® 2 system (bioMérieux, Marcy l’Etoile, France; AST-GN65 card) at the Laboratory of Mabaret El-Asafra, Alexandria, Egypt based on the manufacturer’s guidelines. The virulence factors-encoding genes presence; namely Shiga toxin 1 and 2 (stx1 and stx2) were detected by PCR assay as previously described by El-Shouny et al. (2018) using specific oligonucleotide primers (Table 2). PCR was performed in a reaction mixture (25 μl) containing primers (10 mM), buffer (1X), MgCl2 (25 mM), dNTPs (10 mM), Taq polymerase (5U) and sterile distilled water in a final volume of 25 μl. PCR amplification conditions were denaturation step at 95ºC for 10 min, 35 amplification cycles at 95 ºC for 45 s, annealing temperature depending on each primer pair of resistance-encoding genes and virulence factors-encoding genes for 30 s and 72 ºC for 45 s with a final elongation step at 72 ºC for 10 min. Amplicons were revealed by gel electrophoresis on a 1.2% agarose stained with ethidium bromide at 100 V for 30 min.
Synthesis of ZnO2-NPs
With a transition temperature (211°C) and size (15-25 nm), a pure phase of ZnO2-NPs was obtained as a generous gift from Prof. Dr. Sameh Samir Ali (Biofuel Institute, Jiangsu University, China). ZnO2-NPs were synthesized, according to Ali et al. (2017). Briefly, a mix of 10 ml of NH4OH and 20 ml of 0.1 M of zinc acetate dihydrate was added to homogenized acetone (70 ml) and glycerol (3 g). Forty ml of H2O2 (40%) was added to the prepared solution with constant stirring at 25°C for 30 min. The precipitate was centrifuged and washed with distilled water.
Table 2: Oligodeoxynucleotide primers used for PCR amplification.
| Target gene | Primer sequences | References |
| stx1 | ACACTGGATGATCTCAGTGG | Dipineto et al., 2006 |
| CTGAATCCCCCTCCATTATG | ||
| stx2 | CCATGACAACGGACAGCAGTT | |
| CCTGTCAACTGAGCAGCACTTTG |
Antimicrobial activity of ZnO2-NPs
Antimicrobial activity of ZnO2-NPs was assessed by the preparation of various concentrations (50, 100, 150, and 200 µg/ml) of a stock solution (2 g/ml). Filter paper discs (6 mm) were dipped in the prepared solutions and placed on Muller Hinton agar plates that were previously inoculated with the bacterial isolates. The plates were incubated at 37 °C for 4 days. Distilled water was used as a control for the antibacterial activity. Triplicates were maintained, and the mean values and standard error were estimated for all the inoculated plates, as reported previously (Ali et al., 2017; CLSI, 2017).
Statistical analysis
The bacterial count was transformed using Log10 before the statistical analysis. The obtained data were analyzed statistically using ANOVA (one-way) and t-test (using NCSS 2019 software. All analyses at p ≤ 0.05 were considered significant.
RESULTS
Biochemical identification of E. coli
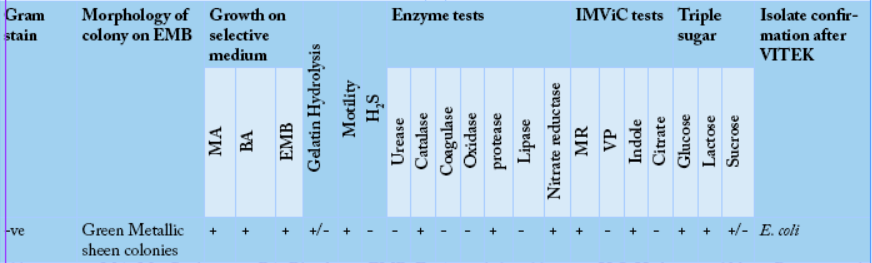
The biochemical identification for Gram-negative bacteria (E. coli) was performed for all isolates. The growth of suspected bacterial isolates on different media showed a good growth rate on MacConkey agar and blood agar. However, the growth was variable on EMB agar. The growth of the bacterial strains on EMB revealed green metallic sheen colonies. The selected bacterial isolates were subjected to biochemical identification which exhibited that the bacterial isolates were motile and showed a negative result when subjected to hydrolysis and H2S test. However, the gelatinase test showed variable results. The enzymatic reaction tests showed positive results for
Abbreviations: MA: MacConkey agar, BA: Blood agar, EMB: Eosin methelene blue agar, H2S: Hydrogen sulfide; +: Positive result; -: Negative results and +/-: Variable results.
protease, nitrate reductase, and catalase. However, urease,
oxidase, coagulase, and lipase tests showed negative results. The IMViC tests for the bacterial isolates showed positive results for MR and indole tests. However, the VP and citrate showed negative results. Triple sugar fermentation was evident for glucose, lactose, and sucrose (Table 3). The biochemically tested bacterial isolates were later confirmed by VITEK® 2 system.
Microbiological quality assessment
The obtained data as shown in Table 4, revealed a higher count of Enterobacteriaceae in fresh meat (5.54 log10 CFU/g) than chilled (3.03 log10 CFU/g) and frozen (1.72 log10 CFU/g). The total coliform count of fresh meat (3.81 log10 CFU/g) was also significantly higher (P ≤ 0.05) than chilled meat (2.12 log10 CFU/g) and frozen meat (1.23 log10 CFU/g).
Table 4: Analytical results of microbiological quality evaluation of chicken carcasses.
| Chicken meat cuts-up |
E. coli (n=10) |
||
| Fresh | Chilled | Frozen | |
| Breast |
5.20±0.52a |
3.27±0.20a |
2.11±0.11a |
| Thigh |
5.51±0.23a |
3.12±0.35ab |
1.17±0.35b |
| Liver |
4.92±0.29a |
2.99±0.25b |
1.51±0.26b |
| Gizzard |
6.51±0.33b |
2.75±0.28c |
2.07±0.15a |
| Average | 5.54 | 3.03 | 1.72 |
| P-value | 0.884 | 0.268 | 0.721 |
| F value | 0.125 | 1.531 | 0.34 |
Values were measured by log10 CFU/g and represented as the mean of three replicates (mean±SD).
Antimicrobial susceptibility test
The effectiveness of gentamicin and chloramphenicol was closely related to the maximum antibacterial activity against E. coli. However, showing minimum antibacterial activity, vancomycin, and tetracyclin were closely related. One percent (3/262) of all isolates was susceptible to all antimicrobial agents, while 81% (212/262) isolates were MDR strains. The resistance rates were maximum for vancomycin (99%) and tetracycline (96%), while it was lowest for gentamicin (7.2%) and chloramphenicol (12.6%). Hence, gentamicin showed efficiency against E. coli isolates. The remaining antimicrobial agents showed varying resistance that ranged from 24-73% as shown in Table 5. and isolates were selected as PDR E. coli strains.
Table 5: Antibiotic susceptibility of all tested E. coli isolates.
| Antibiotic class | Antimicrobial agent | Sensitive | Intermediate | Resistant | |||
| No. | (%) | No. | (%) | No. | (%) | ||
| Aminoglycosides | Gentamicin (CN) | 243 | 92.7 | 4 | 1.5 | 15 | 5.7 |
| Carbapenems | Imipenem (IPM) | 199 | 76.0 | 7 | 2.7 | 56 | 21.4 |
| Cephalosporin | Cephalothin (KF) | 131 | 50.0 | 6 | 2.3 | 125 | 47.7 |
| Fluoroquinolones | Ciprofloxacin (CIP) | 136 | 51. | 6 | 2.3 | 120 | 45.8 |
| Glycopeptides | Vancomycin (VA) | 3 | 1.1 | 11 | 4.2 | 248 | 94.7 |
| Lipopeptides | Colistin sulphate (CT) | 179 | 68.3 | 8 | 3.1 | 75 | 28.6 |
| Penicillins | Ampicillin (AMP) | 71 | 27.1 | 11 | 4.2 | 180 | 68.7 |
| Phenicols | Chloramphenicol (C) | 229 | 87.4 | 4 | 1.5 | 29 | 11.1 |
| Sulphonamide | Trimethoprim/sulfamethoxazole (SXT) | 158 | 60.3 | 5 | 1.9 | 99 | 37.8 |
| Tetracyclines | Tetracycline (TE) | 10 | 3.8 | 12 | 4.6 | 240 | 91.6 |
|
β-Lactam |
Amoxycillin/clavulanic acid (AMC) | 135 | 51.5 | 6 | 2.3 | 121 | 46.2 |
Phenogenotypic characterization of virulence factors
The phenotypic characterization of the selected PDR E. coli strains revealed a100% positivity to β‐hemolysins, while 86% of the isolates were positive to the cell surface hydrophobicity (CSH) test. However, 57.1% Of PDR strains were positive for MRHA and gelatinase enzymes. Two E. coli isolates (ECDMGF6 and ECKFGF5) were positive for all of the virulence factors tested. However, stx1 and stx2 were absent in these isolates. The investigation of Shiga toxins producing E. coli strains revealed the presence of stx2 in two strains of E. coli (ECGHBF1 and ECMFLF4) (Table 6).
Table 6: Phenogenotypic characterization of PDR E. coli isolates virulence factors.
| PDR isolate code | Virulence factors | |||||
| Phenotypic characterization | Genotypic characterization | |||||
| Gelatinase | CSH | MRHA |
β‐hemolysins |
stx1 | stx2 | |
| ECGHBF1 | - | + | + | + | - | + |
| ECGHTC3 | + | - | + | + | - | - |
| ECDKLC2 | - | + | - | + | - | - |
| ECMFLF4 | + | + | - | + | - | + |
| ECGHVF2 | - | + | - | + | - | - |
| ECDMGF6 | + | + | + | + | - | - |
| ECKFGF5 | + | + | + | + | - | - |
Antimicrobial activity of ZnO2-NPs
At a concentration of 50 µg/ml of ZnO2-NPs, E. coli showed lower inhibition zone that ranged from 0.0-20.0 mm. At 100 and 150 µg/ml, all E.coli strains showed susceptibility to ZnO2-NPs. A high dose of ZnO2-NPs (200 µg/ml) showed an outstanding susceptibility of all bacterial strains. The minimum inhibitory concentration (MIC50) of E. coli isolates ranged from 6.25-150 µg/ml. However, minimum bactericidal concentration (MBC) ranged from 12.5-300 µg/ml (Table 7).
Table 7: Zinc peroxide nanoparticles activity against PDR strains.
| Isolate code | Concentrations of ZnO2-NPs (µg/ml) | MIC50 | MBC | |||
| 50 | 100 | 150 | 200 | |||
| ECGHBF1 | 17.5±1.0 | 20.5±1.5 | 25.0±1.5 | 27.0±1.0 | 25 | 50 |
| ECGHTC3 | 15.5±1.0 | 18.5±2.0 | 20.5±0.5 | 23.5±0.5 | 12.5 | 50 |
| ECDKLC2 | 0.0±0.0 | 15.5±2.0 | 18.0±1.0 | 20.0±1.0 | 150 | 300 |
| ECMFLF4 | 15.5±0.5 | 16.5±1.0 | 22.0±1.0 | 25.5±1.5 | 25 | 50 |
| ECGHVF2 | 16.5±1.0 | 18.5±1.0 | 19.5±1.0 | 21.0±1.5 | 25 | 50 |
| ECDMGF6 | 17.0±1.0 | 19.0±0.5 | 21.5±0.5 | 25.5±1.5 | 12.5 | 25 |
| ECKFGF5 | 20.0±1.5 | 22.0±1.0 | 25.0±2.5 | 28.0±2.0 | 6.25 | 12.5 |
| P- value | 0.97 | 0.92 | 0.999 | 0.973 | ND | ND |
| F | 0.030 | 0.083 | 0.001 | 0.027 | ND | ND |
DISCUSSION
Our data indicated the presence of E. coli in meat, which is in agreement with previous findings. For instance, Abdellah et al. (2013) reported the presence of E. coli while surveying the microbiological quality of 96 samples of turkey meat in Morocco. Furthermore, Polpakdee and Angkititrakul (2015) studied and reported the presence of S. aureus, E. coli, and Salmonella spp. in cooked and raw chicken meat in Thailand.
Our data revealed an increased E. coli average count in fresh cuts-up samples (5.9 log10 CFU/g) than chilled and frozen cuts-up (4.46 - 3.49 log10 CFU/g). We observed a higher count than previous reports. For example, Adu-Gyamfi et al. (2012) documented the microbiological quality of chicken at different retail stores in Accra, Ghana. They found varying counts of E. coli such as 1.27, 2.59, and 2.74 log10 CFU/g in chicken meat, where all of them were lower than the ones observed in the current study. In another study, Pissol et al. (2013) estimated E. coli contamination in 161 carcasses originating from Brazil and found a reduction of about three log10 for the carcasses with previous contamination. These results are in agreement with those of Odwar et al. (2014). They noted 78% contamination rate of chicken samples by E. coli, where was above the acceptable range conferred by the Hazard Analysis and Critical Control Point system (HACCP), Food and Agriculture Organization (FAO) and the Codex Alimentarius Commission (3.911 and 5.0261 Log10 CFU/ml respectively).
Our obtained data were in agreement with Kibret and Abera (2011) who reported that gentamicin was effective against 79.6% of E. coli isolates. Alharbi et al. (2018) tested the zoonotic MDR E. coli and reported that 100% of isolates were sensitive against ciprofloxacin, gentamicin, and imipenem. However, Younis et al. (2017) revealed a high resistance of E. coli to penicillin (100%) and low resistance to norfloxacin (36.9%) and chloramphenicol (30%).
Shiga toxins were widely spread in the meat especially in minced meat. The capability of E. coli to induce infection in humans is based on the production of Shiga toxins (Leotta et al., 2008). Shiga toxin-producing E. coli can cause food-borne infection besides the severe fatal illnesses in humans, such as hemorrhagic colitis and hemolytic uremic syndrome which suggested being the major cause of acute renal failure in children (Prendergast et al., 2011). In our study, genotypic characterization revealed that stx1 was absent in all PDR E. coli strains, while the stx2 gene was found in 29% of isolates. The molecular identification of PDR strains showed the presence of E. coli O157: H7 ATCC 43888, 90-9133, EMB 210, PS8, 963, NCYU-21-79, and 4928STDY7071340. In this context, Younis et al. (2017) noted most isolates as E. coli strains serotype O78 and O2. Serologically, E. coli isolates belonging to specific O serogroups.
The antimicrobial activity of ZnO2-NPs was investigated against PDR E.coli isolates in our study and the obtained results showed an outstanding activity. Also, Ali et al. (2020) reported that the activity of metal oxide nanoparticles (ZnO2-NPs, ZnO-NPs, and TiO2-NPs) was tested against E. coli isolates and its efficiency estimated by 72.7, 52.7, and 43.6%, respectively. On the other hand, ZnO-NPs activity against S. aureus (Gram-positive bacteria) was demonstrated by Narasimha et al. (2014) and reported an excellent antibacterial effect. The nanoparticles destroy the bacterial cell wall membrane by changing their membrane permeability and stimulating oxidative stress. Reddy et al. (2014) demonstrated that the nanoparticles decline the invasion and internalization by non-phagocytic cells. Hsueh et al. (2015) reported the accumulation of nanoparticles in the cytoplasm or on the outer membranes of the bacteria, causing cell death. Also, Hoseinzadeh et al. (2017) found that the electrostatic attraction occurred when nanoparticles charges are positive, stimulate the accumulation into the negatively charged bacterial cell membrane.
Conclusion
Food containing multidrug-resistant and multivirulence producing E. coli especially poultry meat that could serve as a source of transmitting MDR genes to humans became a serious threat. ZnO2-NPs have displayed outstanding antibacterial activity against zoonotic PDR E. coli isolates, and therefore could be promising and potential metal oxide nanoparticles for food safety applications.
ACKNOWLEDGMENTS
The authors grateful thank Prof. Dr. Sameh Samir Ali, Associate Professor of microbiology, Biofuel institute,Jiangsu University, China for his valuable assistance during the preparation of this manuscript.
Authors Contribution
EAA designed the experiment and wrote the manuscript. AEA, MAH, TSE wrote the manuscript. TSE analysed the data AEA, MAH reviewed the manuscript. AEA edited and approved the final manuscript.
Conflict of interest
The authors have declared no conflict of interest.
References