Advances in Animal and Veterinary Sciences
Research Article
Moringa Oleifera Leaves Extract Ameliorates Melamine-induced Testicular Toxicity in Rats
Mohamed Fouad Mansour1, Ahmed Hamed Arisha2*, Mohamed Al-Gamal1, Rasha Yahia1, Ahmed A. Elsayed3, Saydat Saad1, Khlood M. El-Bohi4
1Department of Biochemistry, Faculty of Veterinary Medicine, Zagazig University, 44519 Zagazig, Egypt; 2Department of Physiology, Faculty of Veterinary Medicine, Zagazig University, 44519 Zagazig, Egypt; 3Photochemistry Department, Industrial Chemical Division, National Research Center, Dokki, Giza, 12622, Egypt; 4Department of Toxicology and Forensic Medicine, Faculty of Veterinary Medicine, Zagazig University, 44519 Zagazig, Egypt.
Mohamed Fouad Mansour and Ahmed Hamed Arisha contributed equally.
Abstract | Testicular toxicity is a significant cause of male infertility. It may occur due to hormonal, nutritional, behavioral, and environmental imbalance. Moringa oleifera, on the other hand, is known to have antioxidant, anti-inflammatory, anti-tumor, anti-hypertensive, anti-diabetic properties. This study assessed the ameliorative effect of Moringa oleifera leaves extract (M/MOLE) on melamine-induced testicular toxicity in rats. Fifty Sprague Dawley (8weeks old) male rats were randomly assigned to group 1 (control), group 2 ( Moringa oleifera leaves extract only), group 3 (administered melamine only), and group 4 (both melamine and M/MOLE). Melamine significantly decreased (P < 0.05) semen quality, testicular weight, gonadal-somatic index, follicle-stimulating hormone (FSH), free testosterone, total testosterone, and an expression of steroidogenic enzyme genes (CYP11A1 and HSD17B3). A concurrent administration of M/MOLE reversed the clinical impact of Melamine treatment (P < 0.05). Histopathological examination revealed an improvement in testicular tissue injury in concurrent M/MOLE treatment groups than the melamine group. In conclusion, MOLE was effective in improving the toxic effects of melamine on testicular tissue in rats.
Keywords | Moringa oleifera leaves, Testicular toxicity, Steroidogenesis, Semen picture
Received | June 10, 2020; Accepted | June 25, 2020; Published | July 10, 2020
*Correspondence | Ahmed Hamed Arisha, Department of Physiology, Faculty of Veterinary Medicine, Zagazig University, 44519 Zagazig, Egypt; Email: vetahmedhamed@zu.edu.eg
Citation | Mansour MF, Arisha AH, Al-Gamal M, Yahia R, Elsayed AA, Saad S, El-Bohi KM (2020). Moringa oleifera leaves extract ameliorates melamine-induced testicular toxicity in rats. Adv. Anim. Vet. Sci. 8(s1): 47-54.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.s1.47.54
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Mansour et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Testicular toxicity accounts for approximately 50% of cases of infertility in men (Agarwal et al., 2015; Inhorn and Patrizio, 2015). The critical factors responsible for testicular toxicity include hormonal, environmental, behavioral, and nutritional imbalances (Sharpe and Franks, 2002). Oxidative stress (OS) is the chief cause of testicular toxicity and male infertility (Agarwal et al., 2014; Aitken, 2016; Aitken et al., 2019; Bui and Sharma, 2018; Wagner et al., 2018). Many factors such as cryptorchidism, testicular torsion, varicocele, hyperthyroidism, infection, physical exertion, hormonal imbalance, the impact of xenobiotics induce the formation of reactive oxygen species (ROS) in the testis (Aitken and Roman, 2008). ROS is one of the principal causes of alteration in glucose homeostasis in testis, especially in terms of lactate production and modulation of LDH activity (Rossi et al., 2016) and apoptosis of germ cell (Rao and Shaha, 2000).
Moringa oleifera (MO), a drumstick or horse-radish tree from Africa and India, possess high nutritional and medicinal effects (Almatrafi et al., 2017; Leone et al., 2016). It is a tropical plant which has been studied intensively for possible human health application (Leone et al., 2016). Leaves are the most utilized part of the moringa characterized by their increased nutritional value (Verma et al., 2009). Recent studies have reported its antioxidant, anti-inflammatory, and anti‐diabetic properties (Zeng et al., 2019).
Following the administration of cyclophosphamide in mice, Moringa oleifera leaf (MOL) has been shown to protect spermatogonial cells and alleviate cellular damage (Nayak et al., 2016). Furthermore, the hexane extract of MOL has been associated with enhanced functions of the seminiferous tubule, testis, epididymis, and seminal vesicle (Cajuday and Pocsidio, 2010b). These effects might correspond to the abundance of bioactive constituents in moringa leaves, particularly the polyphenols (phenolic acids and flavonoids) and isothiocyanates (Waterman et al., 2015). The moringa extract is known to have antioxidant, anti-inflammatory, anti-tumor, anti-hypertensive, anti-pyretic, anti-spasmodic, anti-epileptic, anti-ulcer, cholesterol-lowering, renal diuretic (Sharma et al., 2012) and hepato-protective activities (Huang et al., 2012). It also improves male sexual functions, including libido, sperm quality, and erectile function (Prabsattroo et al., 2012). Though the treatment of male infertility has several management options such as the use of drugs and/ or surgical intervention (Purvis and Christiansen, 1993). We assessed the potential ameliorative effect of melamine, and Moringa oleifera leaves extract (MOLE) on melamine-induced testicular toxicity in rats. It was hypothesized that MOLE possesses the potential ameliorative effect on semen characteristics, antioxidant status, histopathological examination, and steroidogenic gene expression in melamine-induced testicular toxicity in rats.
Materials and Methods
Preparation of ethanolic extracts of moringa oleifera
The leaves obtained from obtained from the Egyptian Scientific Society of Moringa National Research Centre (Dokki, Giza Egypt) were carefully washed and dried at a temperature ranging from (29-35°c) for three weeks, as previously reported (Ugwu et al., 2013). The leaves were pulverized into coarse shapes with a crestor high-velocity milling machine. The coarse format (1000 g) was macerated in absolute ethanol and left to settle down in a standing position for 48h. The extract was filtered, concentrated, and evaporated to dryness using a rotary evaporator. Finally, using a polysaccharide carrier, the concentrated extract was diluted to the volume of one liter and stored at 4°c. Samples of Moringa oleifera ethanolic extracts were subjected to GC–MS analysis as per the method reported previously (Abd-Elhakim et al., 2018).
Experimental design
Fifty healthy male Sprague Dawley (8 weeks old) rats obtained from the laboratory animal facility of the Faculty of Veterinary Medicine at Zagazig University were randomly assigned, four groups. Group 1 (10 rats, control) received distilled water (1 ml). Group 2 (10 rats) administered Moringa oleifera (800 mg/kg.bw) (Abd-Elhakim et al., 2018). Group 3 (15 rats) administered melamine (800 mg/kg.bw) (Early et al., 2013), and Group 4 (15 rats) administered melamine (800 mg/kg.bw) an hour before Moringa oleifera (800 mg/kg.bw). The whole experiment was extended to a period of six weeks. All experimental procedures were approved by the Zagazig University Institutional Animal Care and Use Committee (acceptance number ZU-IACUC / 2/ F/ 163/ 2019).
Sample collection
At the end of six weeks, rats were sacrificed by decapitation, followed by exsanguination. Blood samples from each rat were collected, left for one and a half hours, and centrifuged at 3,500 rpm for 15 min. The separated serum was maintained at -20 °C until analyzed. Both testes were dissected free from adhering tissue and weighed. A 30 mg of testicular tissue was dissected and stored in -80oC for the real-time PCR procedures. One gram of testicular tissue per rat was homogenized for antioxidant measurement, while the rest of the testes was preserved in 10% neutral buffered formalin for histopathological examination. Absolute and relative weight measurements were recorded according to previous reports (Arisha and Moustafa, 2019; Khamis et al., 2020).
Biochemical and hormonal assays
Previously reported biochemical and hormonal tests were used in the current study (Arisha and Moustafa, 2019; Khamis et al., 2020). Antioxidant enzyme concentration was assessed through commercial kits (Biodiagnostic Company, Dokki, Giza, Egypt) as per manufacturer instructions. The ELISA kits were used serum hormonal levels (MyBioSource, San Diego, CA, USA) where optical density was measured by a plate reader (DNM–9602; Beijing Perlong Medical Instrument Ltd., China).
Semen evaluation and sperm parameters
Utilizing the caudal part of epididymis, different semen parameters have been reported previously (Arisha et al., 2019; Arisha and Moustafa, 2019; Hussein et al., 2019; Khamis et al., 2020). We followed similar procedures to assess sperm motility, the ratio of live-to-dead sperm count, sperm cell concentration, and the percentage of sperm abnormalities.
Table 1: Primers Sequences used for real time PCR.
|
Forward primer (5′–3′) |
Reverse primer (5′–3′) |
Accession No | Product size | |
| CYP11A1 | AAGTATCCGTGATGTGGG | TCATACAGTGTCGCCTTTTCT | NM_017286.3 | 127 |
| HSD17B3 | AGTGTGTGAGGTTCTCCCGGTACCT | TACAACATTGAGTCCATGTCTGGCCAG | NM_054007.1 | 161 |
| CYP19A1 | GCTGAGAGACGTGGAGACCTG | CTCTGTCACCAACAACAGTGTGG | NM_017085.2 | 178 |
| Gapdh | GGCACAGTCAAGGCTGAGAATG | ATGGTGGTGAAGACGCCAGTA | NM_017008.4 | 143 |
Table 2: Effect of orally administered melamine and /or Moringa oleifera leaves extract (M/MOLE) on semen parameters. Values are mean ± SEM of 8-10 rat per group.
| Control | Moringa | Melamine | Co-treated | |
| Sperm motility (%) |
85.33 ± 2.6a |
94.67 ± 2.91a |
51.67 ± 7.26b |
88.33 ± 4.41a |
|
Sperm count (sperm cell concentration/ml x 125 x 104) |
86.33 ± 5.04b |
109.67 ± 5.81a |
44 ± 3.61c |
77.33 ± 6.17b |
| Sperm abnormalities (%) |
11.67 ± 2.4bc |
7.33 ± 1.2b |
38.33 ± 5.21a |
21.33 ± 4.33b |
abcMeans bearing different superscripts were significantly different at P < 0.05.
Real-time PCR analysis
We followed a previously reported real-time PCR analysis for analyzing the expression of desired genes (Arisha et al., 2019; Khamis et al., 2020). Using primers and probes (Table 1), the assay was constituted with 5X HOT FIREPol EvaGreen qPCR Mix Plus (Solis BioDyne, Tartu, Estonia) as per manufacturer’s instructions and performed in an Mx3005P Real-Time PCR System (Agilent Stratagene, USA). The relative expression of each gene normalized to the housekeeping GAPDH, and relative to control, was reported as fold change by 2−ΔΔCT (Livak and Schmittgen, 2001).
Histopathological examination
Testicular specimens were embedded in paraffin, sectioned into 5 µm, stained with hematoxylin and eosin (H and E) dyes (Layton and Suvarna, 2013), and examined using light microscope following previous reports (Alam et al., 2019; Arisha et al., 2019; Arisha and Moustafa, 2019; Hussein et al., 2019; Khamis et al., 2020).
Statistical analysis
Statistical analysis was carried out using one-way ANOVA by SPSS 24 (SPSS, Chicago, Ill). Data were expressed as Mean ± SEM, and P< 0.05 was considered statistically significant.
Results
Effect of oral administration of melamine and/or moringa oleifera leaves extract (M/MOLE) on semen parameters and gonadal-somatic index
The sperm motility, count, testicular weight, gonadal-somatic index, and sperm abnormalities were significantly decreased in the melamine group than control (P < 0.05). It was returned to the normal level in the group that had administered melamine, and Moringa oleifera leaves extract (M/MOLE) (P < 0.05) (Table 1). A decrease in testicular weight and the gonadal-somatic index was significantly increased in M/MOLE group compared to the melamine group (P < 0.05) (Figure 1).
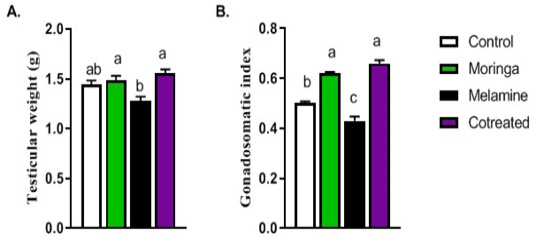
Figure 1: Effect of orally administered melamine and /or Moringa oleifera leaves extract (M/MOLE) on testicular weight and gonadosomatic index (a-b). Values are expressed as mean ± SEM of 8-10 rat/group. Means with different superscripts are significantly different at P < 0.05.
Effect of oral administration of melamine and/or moringa oleifera leaves extract (M/MOLE) on serum reproductive hormones
Figure 2 shows hormonal measurements (FSH, LH, free testosterone, and total testosterone) that were significantly decreased in melamine group than control (P < 0.05). These decreasing levels increased substantially in the M/MOLE group compared to the melamine treated group (P < 0.05). Moreover, results indicated that the estradiol level was significantly increased in the melamine treated group than control (P < 0.05). However, M/MOLE significantly decreased this level to normal (P < 0.05) (Figure 2).
Effect of oral administration of melamine and/or moringa oleifera leaves extract (M/MOLE) on mRNA expression of steroidogenic enzymes
Figure 3 described the transcriptional level of steroidogenic enzymes such as CYP11A1 and HSD17B3 genes. A significant decrease in their mRNA expression was observed in the melamine group than control (P < 0.05). However, this decreased in expression increased significantly in the M/MOLE group than the melamine group (P < 0.05). A significant increase in the expression of the aromatase gene (CYP19A1) was observed in the melamine group than control (P < 0.05), which get decreased significantly in M/MOLE group than melamine group (P < 0.05) (Figure 3).
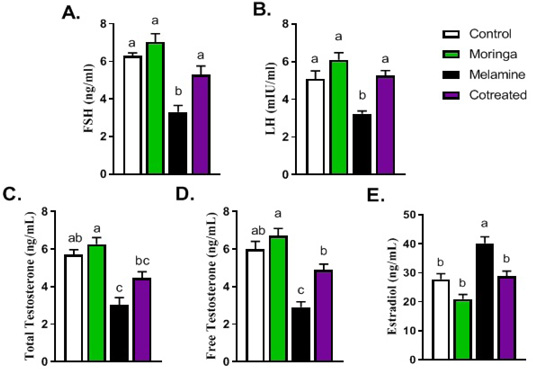
Figure 2: Effect of orally administered melamine and /or Moringa oleifera leaves extract (M/MOLE) on serum levels of gonadotropins and sex steroids (A-E). Values are expressed as mean ± SEM of 8-10 rat/group. Means with different superscripts are significantly different at P < 0.05.
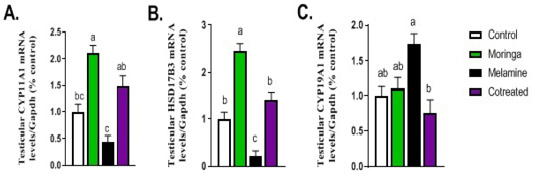
Figure 3: Effect of orally administered melamine and /or Moringa oleifera leaves extract (M/MOLE) on testicular mRNA expression of some steroidogenesis enzymes (A-C). Values are expressed as mean ± SEM of 8-10 rat/group. Means with different superscripts are significantly different at P < 0.05.
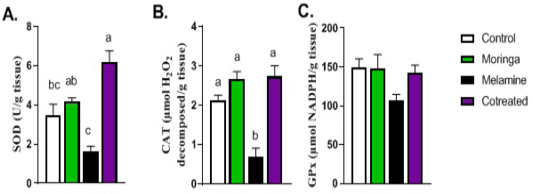
Figure 4: Effect of orally administered melamine and /or Moringa oleifera leaves extract (M/MOLE) on testicular antioxidant defense. Values are expressed as mean ± SEM of 8-10 rat/group. Means with different superscripts are significantly different at P < 0.05.
Effect of oral administration of melamine and/or moringa oleifera leaves extract (M/MOLE) on oxidative stress markers
Regarding the antioxidant status in the testis, the levels of SOD and catalase antioxidant enzymes showed a significant decrease in the melamine group than control (P < 0.05). These decreasing levels were significantly increased in M/MOLE group compared to melamine (P < 0.05). No significant alterations were noticed for the glutathione peroxidase level between the different experimental groups (Figure 4).
Effect of oral administration of melamine and/or moringa oleifera leaves extract (M/MOLE) on testicular morphology
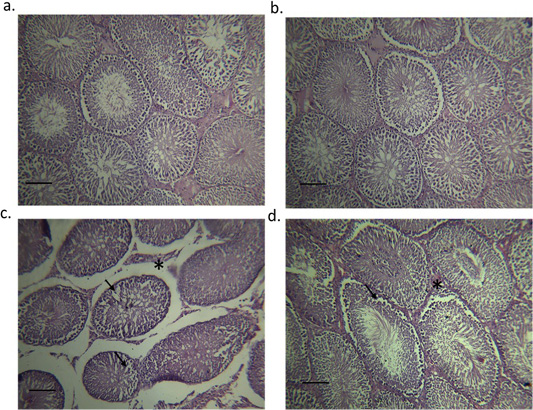
Figure 5 shows the testicular histology of adult male rats originating from different experimental groups. Sections from the testes of control (Figure 5A) and moringa treated group (Figure 5B) showed the normal histological structure of the seminiferous tubules. Melamine group showed a disturbance in the typical architecture, structural changes in the seminiferous tubules, and a focal separation of the basement membrane from the overlying disorganized and vacuolized germinal epithelium (Figure 5C). On the other hand, M/MOLE treated group showed moderate inter-tubular edema as well as mild disorganized and vacuolized germinal epithelium (Figure 5D).
(a) the control group; seminiferous tubules filled with spermatozoa (indicated by asterisk), (b) Moringa treated group; seminiferous tubules filled with spermatozoa (indicated by asterisk), (c) testes of the orally administered melamine group showing disturbance in the normal architecture, structural changes in the seminiferous tubules and a focal separation of the basement membrane from the overlying disorganized and vacuolized germinal epithelium (indicated by arrows) and (d) Testes of the concurrent M/MOLE treated group showing moderate intertubular edema (indicated by asterisk) as well as mild disorganized and vacuolized germinal epithelium (indicated by arrows).
Discussion
Melamine is a widely used chemical in the industry. There is a paucity of data pertaining to acute and chronic exposure of melamine in humans and animals (Skinner et al., 2010). Melamine (MA) exposure causes reproductive toxicity in males; however, the underlying mechanism is yet unclear (Chang et al., 2018). On the other hand, Moringa oleifera has actively been used in the treatment of certain diseases such as hepatic steatosis, insulin resistance, and cardiovascular disease (Almatrafi et al., 2017). Moringa oleifera encompasses certain plant pigments that possess a powerful antioxidant ability (Maida et al., 2005). It has anti-hypertensive, antioxidant, anti-inflammatory, anti-diabetic, cholesterol-lowering (Sharma et al., 2012) and hepatoprotective activities (Huang et al., 2012). Therefore, our objective was to assess the potential ameliorative effect of Moringa oleifera leaves extract (M/MOLE) on melamine-induced testicular toxicity in rats.
Evaluation of several semen parameters, including sperm count, motility, and abnormality index, is essential for proper assessment of male fertility (Komiya et al., 2013). These parameters reflect any physical or chemical alterations that may affect testicular tissue (Atli et al., 2016). Our data showed that sperm motility and count were significantly decreased in the melamine group than the control. Melamine showed reproductive toxicity against male mice since it damaged testicular tissue structure, induced abnormal formation, and maturation of sperms and lowered semen quality and quantity leading to the enhanced apoptotic activity of spermatogenic cells (Huang et al., 2018). Our data indicated that the decrease in sperm motility and count returned to the normal level in M/MOLE group. These results are consistent with those reported previously (Sadek, 2014), where the author reported significant improvement in testicular toxicity effects upon concurrent administration of chromium and Moringa oleifera extract. The results indicated significantly increased sperm abnormalities in the melamine treated group than the control. However, M/MOLE group significantly improved these abnormalities compared to the melamine group. These results may be attributed to the antioxidant’s rich characteristics of moringa leaves, which might influence the epididymal antioxidant system and improve the spermatogenic process. Following proper treatment with moringa or its isolated phytochemicals, several studies have shown improved levels of several detoxication biomarkers and antioxidant enzymes previously (Ashok and Pari, 2003; Faizi et al., 1994). Another study reported an improved morphology of sperms upon concurrent administration of chromium and Moringa oleifera extract than chromium induced-testicular toxicity group (Sadek, 2014).
Testicular weight and gonadal-somatic index were significantly decreased in the melamine group than the control. This decrease was significantly increased in M/MOLE group compared to melamine. Testosterone synthesized and produced by testicular interstitial cells is controlled via a feedback mechanism under the hypothalamic-pituitary-gonadal axis control (Chang et al., 2014). Regarding hormonal measurements in our experiment, FSH, LH, free testosterone, and total testosterone were significantly decreased in the melamine group than the control. However, it was found otherwise in M/MOLE group compared to melamine treated group. These results are in agreement with those reported previously (El-Sheikh et al., 2016), who revealed an increase in the level of serum testosterone, LH and FSH in male Wister rats after administration of moringa. Treatment with moringa significantly increased the levels of three sex hormones to the normal values compared to diabetic group (Ebong, 2014). An increased testosterone level in M/MOLE group may be attributed to increasing serum LH levels. LH binds to its receptor in the testicular interstitial cells to improve testosterone synthesis and subsequent elevation in the serum (Huang et al., 2018). On the other hand, upon administration of hexane fraction from the leaves of M. oleifera, (Cajuday and Pocsidio, 2010a) reported an enhancement in testis, epididymis, and seminal vesicle with no alteration in serum FSH and LH levels. Our results indicated a significant increase in estradiol levels in the melamine treated group than the control. However, M/MOLE group revealed otherwise where it was returned to a normal physiological level. These observations are consistent with those reported previously (Zeng et al., 2019), where no change in serum estradiol level was observed in female mice fed with Moringa oleifera leaves.
To our knowledge, we are the first who reported the effect of M/MOLE on the expression of mRNAs (CYP11A1, HSD17B3, and CYP19A1). The expression of steroidogenic hormones, including CYP11A1 and HSD17B3 genes, showed a significant decrease in the expression of testicular mRNA in the melamine group than the control. These decreased levels were increased significantly in the M/MOLE group compared to the melamine treated group. This result was further confirmed by an increase in expression of both genes in testicular tissue of male rats that were administered MOLE alone. These results indicate that MOLE may increase the synthesis of steroidogenic hormones in the testis of male rats; however, further studies are needed to ascertain our understanding of the underlying mechanism. The expression of the aromatase gene (CYP19A1) showed a significant increase in the melamine group than the control. This increased expression was decreased significantly in the M/MOLE group compared to the melamine treated group. This indicates the potential role of Moringa oleifera leaves in maintaining the normal level of aromatization of testosterone into estrogen in testis. Regarding antioxidant status in the testis, the levels of SOD and catalase antioxidant enzymes showed a significant decrease in the melamine group than the control. (Huang et al., 2018) reported the reproductive toxicity of melamine in male mice with a significant reduction in sperm quality and antioxidant capacity. Such a decreased antioxidants level increased in M/MOLE group compared to the melamine treated group. Contrary to this, (Zeng et al., 2019) reported that no change in serum superoxide dismutase (SOD) level in mice fed with Moringa oleifera leaves. Low concentrations of cytoplasmic scavenging enzymes in the sperm have been reported where, following moringa administration, elevated levels of the antioxidant enzymatic system can improve the reproductive process (D’Cruz S and Mathur, 2005). No significant alterations in the glutathione peroxidase level were detected between the different experimental groups. These observations are consistent with those reported previously where no change in serum glutathione peroxidase was observed in mice fed with Moringa oleifera leaves (Zeng et al., 2019).
Histopathological examination showed abnormal architecture, structural changes in the seminiferous tubules, and a focal separation of the basement membrane from the overlying disorganized and vacuolized germinal epithelium in the melamine group. Melamine has been reported to impact mouse testicles and induce pathological damage (Crissman et al., 2004). Another study revealed melamine induced reproductive toxicity, abnormal testicular tissue structure, abnormal formation and maturation of sperms, and a lowered semen quality and quantity (Huang et al., 2018). On the other hand, testes of the M/MOLE treated group showed moderate inter-tubular edema and mild disorganized and vacuolized germinal epithelium.
Conclusions
Our study concludes that Moringa oleifera leaves extract (M/MOLE) ameliorate the toxic effect of melamine in rats while reducing the impact of toxicity evidenced by an improvement in semen picture, antioxidant status, histopathological features, and steroidogenic gene expression.
Authors contribution
MA, MFM and AHA conceived the project, researched data, analyzed data and drafted the manuscript. RY, AAS, SS, AHA and KME researched the data and reviewed and edited the manuscript. All authors read and approved the final draft of the manuscript.
Conflict of interest
The authors have declared no conflict of interest.
References