Advances in Animal and Veterinary Sciences
Research Article
Effect of Curcumin-Magnesium Oxide Nanoparticles Conjugate in Type-II Diabetic Rats
Yousef M. Shehata1, Mohamed Fouad Mansour1, Salwa Shadad1, Ahmed Hamed Arisha2*
1Department of Biochemistry, Faculty of Veterinary Medicine, Zagazig University, 44519 Zagazig, Egypt; 2Department of Physiology, Faculty of Veterinary Medicine, Zagazig University, 44519 Zagazig, Egypt.
Abstract | Diabetes results in various complications and 90% of all diabetic cases suffer from type-II diabetes (T2D). Therefore, there is a need to find novel interventions that can protect from diabetic complications and reduce the side effects of currently used antidiabetic drugs. We examined the anti-diabetic effect of Curcumin-Magnesium oxide nanoparticles conjugate (Cur-MgO NPs conj) in streptozotocin (STZ)-induced T2D in male rats while considering hematologic, immunologic, and hepatic metabolic responses. The study included three groups: group 1 (control group), group 2 (STZ-induced T2D), and group 3 (STZ-induced T2D in rats that were orally administered with Cur-MgO NPs conj (5mg/Kg/day for 45 days). Complete blood count, levels of plasma glucose, serum insulin, lipid profile, and hepatic expression of the lipogenic enzymes, namely acetyl-CoA carboxylase (ACC) and fatty acid synthase (FAS) were measured. Histopathological examination of spleen was also conducted. Compared to the control group, a significant elevation in blood glucose and homeostatic model assessment- insulin resistance (HOMA-IR), a substantial reduction in serum insulin level and HOMA-β, an upregulation in hepatic mRNA expression of fatty acid synthase (FAS) and acetyl-CoA carboxylases (ACC), and an altered lipid profile were noticed in STZ-induced T2D group. Oral administration of Cur-MgO NPs conj restored some of the parameters to their physiological levels, including glucose, total cholesterol (TC), and low-density lipoprotein cholesterol (LDL-c). Histopathological examination of the spleen showed abnormalities in lymphoid cells in T2D, while the treated group showed moderate improvement in spleen tissue, and cells were rescued from apoptosis. The data of this study revealed that Cur-MgO NP conj has a potential ameliorative effect on the hematologic, immunologic, and hepatic metabolic alterations in STZ-induced T2D in rats.
Keywords | STZ induced T2D, Nanoparticles, Curcumin, Magnesium
Received | June 10, 2020; Accepted | June 25, 2020; Published | July 10, 2020
*Correspondence | Ahmed Hamed Arisha, Department of Physiology, Faculty of Veterinary Medicine, Zagazig University, 44519 Zagazig, Egypt; Email: vetahmedhamed@zu.edu.eg
Citation | Shehata YM, Mansour MF, Shadad S, Arisha AH (2020). Effect of curcumin-magnesium oxide nanoparticles conjugate in type-II diabetic rats. Adv. Anim. Vet. Sci. 8(s1): 26-33.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.s1.26.33
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Shehata et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Diabetes is a condition that develops when the ability of the body to process blood glucose is impaired. It leads to the development of several complications, such as peripheral vascular disease, heart attack, and stroke. Significant diabetic symptoms include increased fatigue, frequent urination, extreme hunger, and thirst (Westerberg, 2013). Type-II diabetes mellitus (T2DM) is a common metabolic condition characterized by insulin resistance followed by pancreatic islet beta-cell failure leading to hyperglycemia (Asmat et al., 2016). Thus, the development of diabetes is associated with either the inability of the pancreas to produce enough insulin or the lack of cellular response to the insulin produced (Gardner et al., 2007). Type-II diabetes (T2D) is also recognized as adult-onset diabetes or non-insulin dependent diabetes mellitus. It is associated with insulin-resistance of a target organ, which decreases the responsiveness to endogenous and exogenous insulin (Bacha et al., 2010).
T2D is characterized by hyperglycemia, insulin resistance, and gradual loss of β-cell function within the course of the disease (Gurzov et al., 2016). The global incidence of T2D is continuously increased, especially among the young. It accounts for about 90-95% of all diabetic recorded cases (Tsai et al., 2002). New strategies are needed to achieve the management of T2D and avoid its complications. Because of difficulties in using the human model in research, rodent models with T2D are the most widely used simply because they share many similarities with diabetes in humans, including dependence on genetic background, sex, and age of the animal (Ghasemi et al., 2014).
Curcumin (Cur), the main active constituent of turmeric, is a natural compound isolated mainly from Curcuma longa (Basnet and Skalko-Basnet, 2011). Physicochemical properties of curcumin get improved in the form of nanoparticles. With the reduction of the particle size and the formation of an amorphous state with the hydrogen bond, it leads to an increase in the release of the compound, reduces the dose of curcumin, and improve its bioavailability (Kazazis et al., 2014). Magnesium (Mg) is an essential intracellular cation and mineral in the living cells of the human body (Rude and Gruber, 2004). There is a well-known link between Mg-deficiency and T2D (Ramadass et al., 2015). A low Mg-intake and excess urinary excretion are the most important mechanisms leading to Mg depletion in patients with T2D. Mg deficiency affects tyrosine kinase activity, which leads to insulin resistance; however, supplementation increase the enzyme activity (Barbagallo and Dominguez, 2015). Nevertheless, the potential antidiabetic effect of their conjugate remains understudied. We explored the anti-diabetic influence of Curcumin Magnesium Oxide nanoparticles conjugate (Cur-MgO Nps Conj) in T2D rats via studying the hematologic, immunologic, and hepatic metabolic effects.
Materials and Methods
Animals
Healthy male albino rats (n=45, 160±20 gm) were obtained from the animal house, Faculty of Veterinary Medicine, Zagazig University. The animals were grouped and housed in polycarbonate cages. Clean and fresh drinking water was provided ad libitum.
Chemical substances
STZ was purchased from the TOKU-E company, Egypt. Nicotinamide and citrate buffer were purchased from Sigma, Egypt. Cur-MgO NPs conjugate was purchased from NanoTech Egypt for Photo-Electronics Communication Center, Egypt.
Experimental design and induction of type 2 diabetes in rats
Rats were kept for 14 days for acclimatization. Blood glucose was measured at day 15 using a digital glucometer (URight blood glucose meter TD-4251). Following overnight fasting (12 h), 30 randomly selected rats were administered with a single intraperitoneal (i.p) injection of Nicotinamide (110 mg/kg b.w). After 15 min, rats were injected freshly prepared STZ (65 mg/kg B.wt) dissolved in 0.1 M cold citrate buffer (pH 4.5) i.p (Kumar et al., 2011). A 20% glucose solution was offered to animals overnight to avoid drug-induced hypoglycemia. The Control group was i.p injected with the same volume of citrate buffer. After 72 h of STZ administration, blood glucose was measured. Only rats with a glucose level of more than 200 mg/dl were used in the study (Qinna and Badwan, 2015). Rats were distributed into three groups. Group 1 (G1) served as a control that was orally administered with saline. Group 2 (G2) included T2D rats that were orally administered with saline. Group 3 (G3) included T2D rats that were orally administered with Cur-MgO NPs conj (5 mg/kg/day for 45 days). Rats were examined weekly for body weight, and fasting levels of serum glucose and insulin. All animal experiments were conducted in accordance to the procedures approved by Institutional Animal Care and Use Committee (ZU-IACUC/2/F/119/2019) at Zagazig University (IACUC).
Sampling
At the end of the experimental period, rats were sacrificed via cervical decapitation. Blood was collected with and without anticoagulant, and whole blood, serum, and plasma were processed for the different hematological biochemical assays. Liver tissue (50 mg) was collected immediately after death, wrapped in aluminum foil, and placed in a liquid nitrogen container until used for gene expression analysis. The spleen was removed, rinsed with normal saline, and fixed in 10% buffered formalin for histopathology (Alam et al., 2019; Layton and Suvarna, 2013).
Hematological and biochemical analysis
The hematologic analysis was performed as per the method described previously (Alam et al., 2019) using a Hemascreen 18 automatic cell counter (Hospitex, Osmannoro-Sesto Fiorentino, Italy). Following the oxidase method, serum glucose concentration was determined using the Spectrum Diagnostics glucose kit. Serum insulin level was estimated through ELISA kit. The direct enzymatic colorimetric liquid method was used for the determination of HDL-c. LDL-C was calculated with the equation LDL= TC – HDL - (TG/5). The VLDL-c was estimated as per the Friedewald method described previously (Friedewald et al., 1972). Evaluation of triacylglycerol (TAG) was done by the GPO-PAP-enzymatic colorimetric method. Cholesterol liquizyme CHOD-PAP enzymatic colorimetric method was used for the determination of cholesterol. Wherever required, relevant commercial kits were obtained from Egyptian Company for Biotechnology, and manufacturer guidelines were followed.
Histopathological examination
The samples were collected and fixed in 10% buffered neutral formalin before processing, and 5 μm thick paraffin sections were obtained. Hematoxylin and Eosin (H and E) staining was done following the previously described standard protocols for histopathological examination (Layton and Suvarna, 2013) and recently reported (Alam et al., 2019; Hussein et al., 2019).
Relative quantitative RT-PCR analysis
A detailed description of the used protocol has previously been reported (Arisha et al., 2019; Arisha and Moustafa, 2019; Khamis et al., 2020). Using primers (Table 1) and a 5x HOT FIRE Pol EvaGreen qPCR Mix Plus (Solis BioDyne, Tartu, Estonia), the real-time RT-PCR was performed following the manufacturer’s guidelines. The relative expression of each gene normalized to the housekeeping GAPDH, and relative to control, was reported as fold change by 2−ΔΔCT (Livak and Schmittgen, 2001).
Statistical analysis
Data were described as mean ± SEM. One-way ANOVA was applied to compare the means of three groups for the different parameters. After significant ANOVA results, Duncan’s multiple range test as Post hoc test were employed to determine differences among groups. Data were analyzed by SPSS version 24. P < 0.05 was considered significant.
Results
Effect of oral administration of Cur-MgO Nps Conj on the glycemic index and HOMA in T2D rats
A significant increase in the level of plasma glucose was noticed in T2D rats compared to the control group. This increase was normalized after treatment with Cur-MgO NPs conj (Figure 1A). A significant reduction in insulin level was detected in T2D rats compared to the control group. Cur-MgO NPs conj failed to significantly increase the insulin level compared to T2D rats (Figure 1B). Compared to the control group, a significant rise in insulin resistance was indicated by HOMA IR levels and a reduction in HOMA β-cell function in T2D rats. On the other hand, a substantial decrease in HOMA IR levels, as well as an increase in HOMA β-cell function, was observed after treatment with Cur-MgO NPs conj (Figure 1C, D).
Effect of oral administration of Cur-MgO Nps Conj on the hematological parameters in type 2 diabetic rats.
A significant reduction in the level of Hb% in the STZ-induced T2D group was observed than the control group (Table 2). Similarly, paralleled to the diabetic group, a significant increase in Hb% was noticed in the treated group. Neutrophils (band and segmented forms) were significantly reduced in T2D rats compared to the control group. This decrease was normalized considerably after treatment with Cur-MgO Nps conj. Oral administration of Cur-MgO Nps conj significantly decreased by monocyte% (P < 0.05) and increased a total of neutrophils % (P < 0.05).
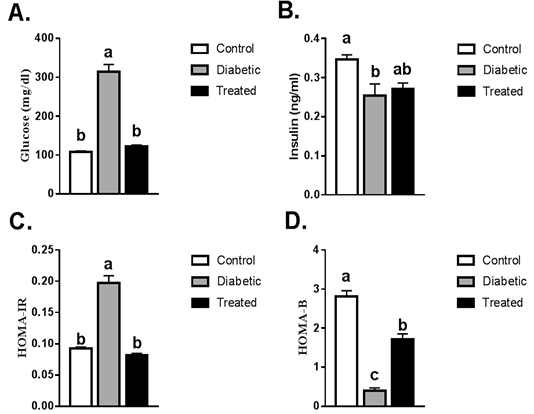
Figure 1: Serum levels of fasting blood glucose, insulin, HOMA-IR and HOMA-β. Values are mean ± SEM (8-10 rats/group). Means with different superscript were statistically different at p ≤ 0.05.
Effect of oral administration of Cur-MgO Nps Conj on the lipid profile in type 2 diabetic rats
The levels of TC, TAG, LDL-c, and VLDL-c were significantly increased while HDL-c levels were significantly reduced in the diabetic group than the control group. Such increases in TC and LDL-c levels were normalized in the treated group compared to the diabetic group. No significant difference was noticed in the levels of HDL-c, TAG, and VLDL-c after treatment as compared to the diabetic group (Figure 2).
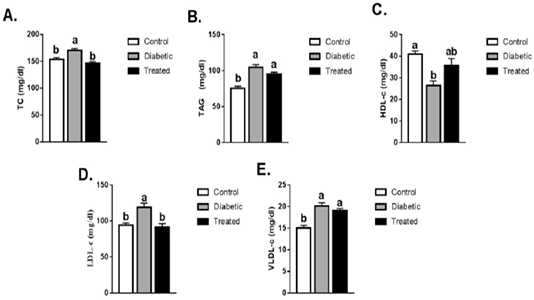
Figure 2: Means ± SEM of lipid profile (TC, TAG, LDL-c, HDL-c, VLDL-c). Values are mean ± SEM (8-10 rats/group. Means with different superscript are statistically different at p ≤ 0.05.
Table 1: Oligonucleotide primers sequences used for real time PCR.
| Gene |
Forward primer (5′–3′) |
Reverse primer (5′–3′) |
| ACC | AACAGTGTACAGCATCGCCA | CATGCCGTAGTGGTTGAGGT |
| FAS | GAGTATACAGCCACCGACCG | AGTTGCACACCACAAGGTCA |
| Gapdh | GGCACAGTCAAGGCTGAGAATG | ATGGTGGTGAAGACGCCAGTA |
Table 2: CBC measurements in the control, diabetic and treated groups.
| Treated group | Diabetic group | Control | |
| 14.54±0.48 | 13.73±0.64 | 14.7±0.43 | Hb (g/dl) |
| 4.99±0.15 | 4.56±0.32 | 4.9±0.16 |
RBCs × 106/cm |
| 6.91±0.58 | 6,17±0.35 | 7.84±0.62 |
WBCs× 103/cmm |
| 1291.13±86.32 | 1145.88±74.03 | 1160.75±71.5 |
Platelet Count×103/cmm |
|
103.60±3.27a |
90.02±5.46b |
105.53±3.21a |
Hb % |
| 44.63±1.28 | 42.49±1.27 | 43.55±0.71 | Haematocrit (P.C.V)% |
| 0.43±0.01 | 0.39±0.02 | 0.40±0.03 | Basophils % |
| 0.22±0.01 | 0.22±0.01 | 0.21±0.01 | Eosinophils % |
|
2.54±0.08a |
2.18±0.07b |
2.86±0.13a |
Band forms % |
|
10.09±0.69a |
7.71±0.77b |
8.25±0.40ab |
Segmented Forms % |
| 84.05±0.63 | 86.09±0.88 | 85.34±0.45 | Lymphocytes % |
|
2.67±0.08b |
2.95±0.07a |
2.95±0.07a |
Monocytes % |
| 89.62±2.04 | 92.33±5.4 | 89.2±1.67 | MCV fl |
| 29,16±0,61 | 29.50±0.94 | 30.03±0.29 | MCH pg |
| 32.65±0.5 | 32.23±0.71 | 33.73±0.59 | MCHC g/dl |
|
11.21±0.6a |
8.75±0.8b |
8.94±0.38b |
Total Neutrophils% |
abcMeans ± SEM at the same row and bearing different superscripts are significantly different at p ≤ 0.05.
Effect of oral administration of Cur-MgO Nps Conj on hepatic expression of ACC and FAS in type 2 diabetic rats
A significant upregulation in the hepatic mRNA expression of ACC and FAS were noticed in STZ-induced T2D rats compared to the control group. This increase was normalized after treatment with Cur-MgO NPs conj (Figure 3).
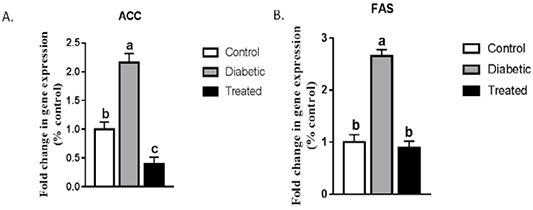
Figure 3: Effect on hepatic mRNA expression of (a) ACC and (b) FAS in adult male rats. Means with different superscripts are significantly different at P < 0.05.
Effect of oral administration of Cur-MgO Nps Conj on the Splenic histopathology
In histopathological examination (Figure 4), splenic tissue showed a typical histological picture as red and white pulps. Prominent lymphoid depletion and damage of lymphoid cells were observed in the diabetic group than the control. Moderate vascular congestion in spleen tissue was detected in the treated group compared to the diabetic group.
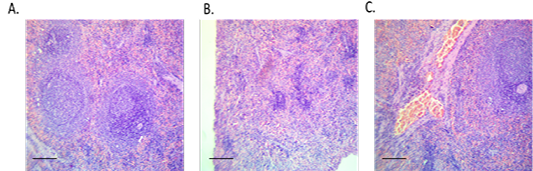
Figure 4: Spleen sections from control, diabetic or treated group. (A) Spleen of control rat showing normal histological picture (red and white pulps), H and E, x10, (B) Spleen of diabetic rat showing lymphoid depletion (shrunken white pulps), H and E, x10 and (C) Spleen of treated rat with the conjugate showing moderate vascular congestion, H and E, x10.
Discussion
Appropriate experimental models are needed to comprehend studies on the pathogenesis and complications of T2D and devise novel strategies for its management (Pari and Rajarajeswari, 2009). Both STZ and NA administration have been used to induce T2DM. It is well-known that STZ damages pancreatic B-cell, while the administration of NA in rats partially prevents the effect of STZ and therefore protect insulin-secreting cells (Brahmanaidu et al., 2017; Kumar et al., 2011). Nanoparticles are tiny materials with size ranging from 1 to 100 nm (Khan et al., 2017).When the size of the material decrease, the surface area increases and results in an enhanced reactivity of the drug. Indeed, the mode of action of the drug is closely related to the particle size (Gera et al., 2017). With the rate of body excretion, it facilitates direct bio-distribution of the particles in the body organs and tissues (Alexis et al., 2008).
A large number of studies have demonstrated the pharmacological and physicochemical effects of curcumin on different types of diseases such as diabetes, cardiovascular diseases, cancer, rheumatoid arthritis, Alzheimer’s disease (Liu et al., 2013). Per os bioavailability of curcumin, nanoparticles exhibited 9-fold improved efficacy compared to generic curcumin (Grama et al., 2013). Nanocurcumin had enhanced stability and solubility of curcumin in aqueous solution and produced an equal diffusion of curcumin than the free form of curcumin (Kumar et al., 2012).
Mg ion is an essential co-factor that is required for more than 300 different enzymatic reactions, including glycolysis (Pasternak et al., 2010). Intracellular Mg is a necessary cofactor for carbohydrate metabolic enzymes, especially in the phosphorylation of the tyrosine kinase of the insulin receptor (Barbagallo and Dominguez, 2007; Saris et al., 2000). There is a well-known link between Mg deficiency and T2D. T2D is often associated with intracellular and extracellular Mg depletion. Hypomagnesaemia is not clinically evident in the T2Dbut exhibit in the chronic latent form of Mg deficiency (Barbagallo et al., 2014; Barbagallo and Dominguez, 2015). Mg deficiency may lead to disorders in the tyrosine kinase activity of the insulin receptor, resulting in post-receptorial insulin resistance and subsequently decreased cellular glucose utilization. This means that, as insulin sensitivity is decreased, the body needs a greater quantity of insulin to process the same amount of glucose (Barbagallo et al., 2003). Magnesium has a significant role in lipid metabolic enzymes, where its supplementation has known to improve insulin sensitivity and lipid profile (Olatunji and Soladoye, 2007). Magnesium oxide nanoparticles can decrease insulin resistance in T2Ds and considered a potential anti-diabetic agent (Jeevanandam et al., 2015). Though need further investigations, It is also known to possess the potential anti-cancer ability (Krishnamoorthy et al., 2012; Martinez-Boubeta et al., 2010).
In this study, after oral supplementation of Cur-MgO Nps conj, blood glucose level returned to a level as that of the control group. This result coincides with another study where a reduced fasting blood glucose levels have been reported with the administration of nano-curcumin (Rahimi et al., 2016). Curcumin inhibits the formation of inflammatory cytokines either directly or through inhibition of nuclear factor- kappa B ( NF-κB ), PGE2, and MAPK, and activates AMPK (Siriwardhana et al., 2013). It also inhibits advanced glycation end products (AGE) production by trapping an AGE precursor-methylglyoxal and increases the number of endogenous antioxidants in the body (Lin et al., 2012). Other studies, which used magnesium supplements in diabetes, have shown that magnesium can decrease blood glucose levels in patients with STZ-induced T2D (Paolisso et al., 1992; Rodríguez-Morán and Guerrero-Romero, 2003; Yokota et al., 2004). Mg is a necessary cofactor for carbohydrate metabolic enzymes, particularly the process of tyrosine-kinase phosphorylation, which is responsible for the insulin receptor (Barbagallo and Dominguez, 2007). Another study reported a reduction in glucose concentrations upon exposure to nano magnesium oxide (Naghsh and Kazemi, 2014).
Nano-curcumin is involved in peroxisome proliferator-activated receptor gamma (PPAR-γ) activity and expression. The PPAR-γ is essential for the activation of the liver enzymes, which are responsible for glycolysis, gluconeogenesis, and lipid metabolic process. These data are in line with our results regarding mRNA expression levels of ACC and FAS. It also can increase lipoprotein lipase (LPL) activity and improve plasma insulin levels (Jazayeri-Tehrani et al., 2019; Seo et al., 2008). In the current study, TC decreased in the treatment group compared to the T2D group. This is not surprising because Nano-curcumin supplementation could significantly reduce the TC level (Rahimi et al., 2016). Serum cholesterol levels in the placebo and treated groups were higher than the control group (Naghsh and Kazemi, 2014). Contradictory to our results, (Rahimi et al., 2016) found a significant reduction in the level of serum TAG before and after the treatment with nano-curcumin. (Qin et al., 2017) showed a significant decrease in serum TAG in patients with metabolic syndrome. (Naghsh and Kazemi, 2014) described that nano magnesium oxide decreases TAG levels in the treatment group than the placebo group, while another study reported a lack of significant difference in serum TAG (Panahi et al., 2017).
Similar to the study results, previous studies reported a decrease in the level of LDL-C after supplementation with nano-curcumin (Rahimi et al., 2016). In patients with metabolic syndrome, a significant decrease in the level of LDL-C has been reported (Qin et al., 2017), while in similar cases, (Panahi et al., 2017) showed a lack of significant changes in its level. A lack of significant change in HDL-C level was also evidenced by the Nano-curcumin supplementation (Rahimi et al., 2016). Similar to LDL-C, in patients with metabolic syndrome, the HDL-C level was not improved significantly (Qin et al., 2017). In another study, though the HDL level was higher in the treatment group than the placebo group, there was no difference with the control group (Naghsh and Kazemi, 2014).
Our study showed a significant increase in the level of Hb% in the treatment group compared to the diabetic group. According to a study (Irace et al., 2011), hemoglobin, hematocrit, and whole blood viscosity were significantly lowered in diabetic retinopathy. Structural modifications of erythrocyte membrane and erythrocyte aggregation in T2DM lead to the short lifespan of RBC and is considered the possible mechanisms for decreased RBC indices. Neutrophils (band andsegmented forms) concentration was reduced significantly in this study, which was normalized in the treatment group. Based on previous results (Milosevic and Panin, 2019), specific parameters of the CBC, such as WBC, platelets, HCT, and PCT, could be valuable indicators to follow T2D and potential complications.
As for histopathological study examination of the spleen is concerned, the control group showed clear and normal red and white pulps. As expected, diabetic rats showed lymphoid depletion (dry white pulps). The conjugate-treated rat showed moderate improvement. These histopathological changes could be illustrated as diabetes-induced oxidative stress, which leads to complications in the spleen. Increasing the level of blood glucose and the intracellular ROS decrease the cellular antioxidant enzymes activity and GSH/GSSG ratio. Subsequently, it resulted in white pulp depletion and damage to the spleen. When treated with Nano-curcumin, there occurred an upregulation of inflammatory chemokines, cytokines, adhesion molecules, and increased translocation of NFκB in the nucleus, resulting in the restoration of these alterations in the spleen (Rashid et al., 2017). Curcumin could have a positive effect on most of the leading features of diabetes, such as hyperglycemia, hyperlipidemia, insulin resistance, islet apoptosis, and necrosis (Wojcik et al., 2018). In conclusion, we proposed a potential synergistic effect among Nano-curcumin and magnesium oxide nanoparticles in the management of T2D.
Acknowledgments
The authors thank Prof. Dr. Mohamed M.M. Metwally (Department of Pathology, Faculty of Veterinary Medicine, Zagazig University) for the histopathological examination of the experiment.
Authors contribution
SS, MFM and AHA conceived the project, researched data, analyzed data and drafted the manuscript. YMS and AHA researched the data and reviewed and edited the manuscript. All authors read and approved the final draft of the manuscript.
Conflict of interest
The authors have declared no conflict of interest.
References





