Advances in Animal and Veterinary Sciences
Research Article
Scanning Electron Microscopic Study of the Anthelmintic Effects of Some Anthelmintic Drugs on Poultry Nematode, Ascaridia galli
Pawi Bawitlung Lalthanpuii, Kholhring Lalchhandama*
Department of Life Sciences, Pachhunga University College, Aizawl 796001, Mizoram, India.
Abstract | Praziquantel/pyrantel pamoate (PZQ/PTP) combination and albendazole (ABZ) are two commercially available anthelmintic drugs most commonly used for the treatment of different helminth infections. Their anthelmintic activity was studied in vitro against the poultry nematode, Ascaridia galli. Although both the drugs were effective, ABZ showed most prominent activity. Scanning electron microscopy of the nematodes revealed structural damaged induced by the drugs. Shrinkage of the cuticle, collapse of the lips, and formation of cuticular folds were the defining anthelmintic effects. Comparison based the drug efficacy and anthelmintic effects indicates that ABZ can be the drug of choice for helminthiasis due to nematodes.
Keywords | Anthelmintic drug, Cuticle, Helminthiasis, Nematode, Scanning electron microscopy
Received | April 13, 2020; Accepted | June 21, 2020; Published | July 07, 2020
*Correspondence | Kholhring Lalchhandama, Department of Life Sciences, Pachhunga University College, Aizawl 796001, Mizoram, India; Email: chhandama@pucollege.edu.in
Citation | Lalthanpuii PB, Lalchhandama K (2020). Scanning electron microscopic study of the anthelmintic effects of some anthelmintic drugs on poultry nematode, Ascaridia galli. Adv. Anim. Vet. Sci. 8(8): 788-793.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.8.788.793
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Lalthanpuii et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Helminthiasis turns out to be the champion of all infectious diseases. It remains the primary factor of morbidity and mortality not only in humans but also in livestock animals. Schistosomiasis, which accounts for 290 million cases in humans as of the latest World Health Organization report (WHO, 2020a), surpasses malaria at 228 million cases as the most prevalent parasitic infection. Nematode infection is estimated to be more prevalent and soars at 1.5 billion cases, i.e. infecting almost a quarter of human population (WHO, 2020b). These nematodes are the major cause of morbidity and morbidity-associated mortality (Kyne et al., 2019). The situation is worsened by rapid and widespread evolution of drug resistance in all major helminths of veterinary importance (Schulz et al., 2018). With reports of total failure of deworming drugs, livestock industry is facing massive economic challenges (Tinkler, 2019).
Albendazole (ABZ) and praziquantel (PZQ) are the most popular anthelmintic drugs for their high efficacy and broad-spectrum activity. They remain the drugs of choice for over three decades for helminthiasis in humans and livestock animals (Hong, 2018). They are also at the frontline medication in debilitating helminth infections such as neurocysticercosis (Garcia et al., 2016), and echinococcosis (Siles-Lucas et al., 2018). However, PZQ is effective against cestodes and trematodes, and not on nematode. In contrast, pyrantel pamoate (PTP) is a nematode-specific drug commonly used in veterinary prescriptions and normally in combination with other drugs. In so far as efficacy is concerned, albendazole alone is effective against all important helminths and produces the highest cure rate (Moser et al., 2017, 2019).
Ascaridia galli Schrank, 1788, is by far the most prevalent and thereby the most important parasite of poultry and wild birds. It is an intestinal nematode causing a range of health problems and pathological complications such as anaemia, droopiness, emaciation and diarrhoea (Brar et al., 2016). The primary effect is loss of nutrient absorption, and severe condition that can lead to death (Gauly et al., 2005). In hens, infection causes loss of appetite, weight loss, reduced egg production and death (Kilpinen, 2005). It also influences increased chances of bacterial infections, which usually add to the gross pathogenicity (Ruhnke et al., 2017; Foka et al., 2019). A. galli are also the only parasites known to infect eggs of their hosts (Bharat et al., 2017). There is no standard anthelmintic drug for the treatment of poultry helminthiasis. It is therefore interesting to study the effects of ABZ and PZQ on this nematode.
MATERIALS AND METHODS
Chemicals and drugs
All reagents used were of standard analytic grades manufacture by HiMedia Laboratories Pvt. Ltd., Mumbai, India. Tetramethylsilane, osmium tetroxide and sodium cacodylate were procured from Merck India, Mumbai, India. Kiwof® (20 mg praziquantel+230 mg pyrantel pamoate) was a product of Sava Medica Limited, Pune, Maharashtra, India. Zenlee® (400 mg albendazole) was a product of UNI-PEX Pharmaceutical Private Limited, New Delhi, India.
In vitro survival test
Live Ascaridia galli Schrank, 1788, were collected from the intestines of freshly slaughtered local poultry, Gallus gallus Linnaeus, 1758. The study approved by the Institutional Ethics Committee of Pachhunga University College (PUC-IAEC-2016-Z2 of 10/08/2016). Anthelmintics were prepared in increasing concentrations of 0.5, 1, 2, 5, 10 and 20 mg/ml in 0.9% neutral phosphate-buffered saline (PBS) supplemented with 1% dimethylsulfoxide (DMSO). The highest dose corresponded to that of albendazole (ABZ) at 10 mg/kg body weight of poultry. Kiwof® was adjusted to 20 mg/ml of pyrantel pamoate (PTP), as praziquantel (PZQ) is not an antinematodal drug. In vitro condition was maintained at 37±1°C in a glass-chambered biological incubator.
Negative control consisted of nematodes maintained in only PBS with DMSO. A set of two worms were tested for each dose, and each test was done in triplicates. Death was recorded when the worms failed to show movement even under agitation in lukewarm PBS (45°C). Data were recorded as statistical means ± standard deviation. Drug efficacy was normalised against the survival time of the control. Significance of the activity was calculated using Student’s t-test. One-way ANOVA was used to compare the efficacy of the two drugs. The level of significance was considered at p < 0.05.
Scanning electron microscopy
Nematodes treated the highest dose, i.e. 20 mg/ml of the anthelmintic drugs were processed for scanning electron microscopy. They were fixed in 4% neutral-buffered formaldehyde at 4°C for 4 hours. Secondary fixation was done in 1% osmium tetroxide at 4°C for 1 hour. The specimens were dehydrated in acetone. They were then treated with tetramethyl silane for 15 minutes. They were then dried in an air-drying chamber, which was maintained at 25°C. The dried specimens were fixed on metal stubs, and then sputter coated with gold in an ion-sputtering chamber (JFC 1100, JEOL Ltd., Tokyo, Japan). Finally, they were examined under a scanning electron microscope (JSM-6360, JEOL Ltd., Tokyo, Japan) with an electron accelerating voltage of 20 kV.
RESULTS
Both PZQ/PTP and ABZ were effective against A. galli and showed dose-dependent efficacy at the concentrations of 0.5, 1, 2, 5, 10 and 20 mg/ml as shown in Table 1. PZQ/PTP took 96.73 ± 1.56, 90.44 ± 1.58, 86.98 1.09, 76.01 ± 1.28, and 62.28 ± 1.11 h, to kill all the nematodes at the different concentrations, while ABZ took 56.27 ± 1.25, 37.90 ± 0.89, 25.17 ± 0.78, 7.96 ± 1.21, 1.77 ± 0.44 h respectively with respect to untreated control. Nematodes in control survived for 226.20 ± 2.73 h. ABZ was significantly (p < 0.05) more effective than PZQ/PTP at all concentrations tested.
For scanning electron microscopy, untreated (control) A. galli and those treated with 20 mg/ml of the drugs were selected. The untreated nematode exhibits a smooth cylindrical body. Figure 1 shows an anterior (cephalic) region of the body showing three lips that surround the mouth. Eye-like projections on the lips are the sensory organs called papillae. The main body indicated that the body surface, called cuticle, is a marked with a series of transverse line or annulations (Figure 2).
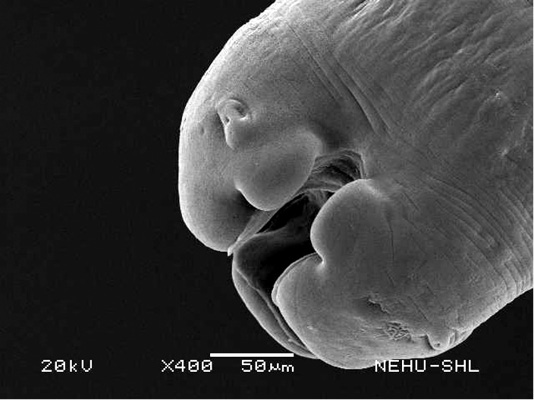
Figure 1: Scanning electron microscopy of the anterior part of an untreated (normal) A. galli. Three lips are seen at the bottom. The central hollow area is the mouth. On two lips are small eye-like papillae.
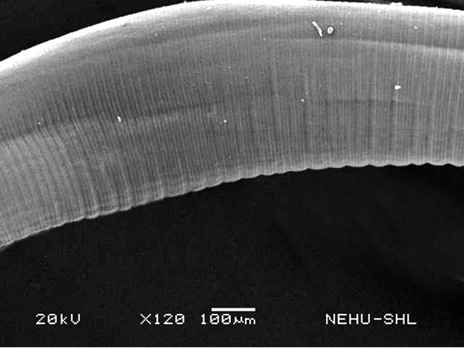
Figure 2: Scanning electron microscopy of the main body of an untreated (normal) A. galli. The cylindrical body consists of a series of transverse lines called annulations.
A. galli treated with PZQ/PTP showed severe shrinkage of the body and collapse of the cuticle. In Figure 3, it is seen that the cuticular disintegration caused deflation of the lips. Shrinkage resulted in formation of irregular folds of the cuticle throughout the body (Figure 4). The posterior tail end is also deflated (Figure 5).

Figure 3: Scanning electron microscopy of the anterior part of A. galli treated with PZQ/PTP. Deflation and distortion of the lips are prominent.
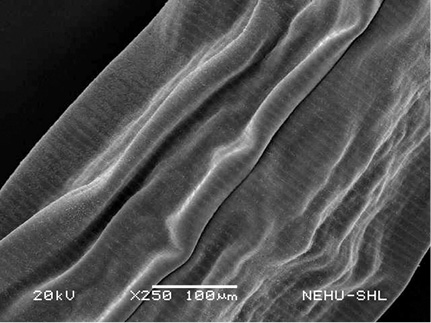
Figure 4: Scanning electron microscopy of the main body of A. galli treated with PZQ/PTP. Large longitudinal folds are seen on the cuticle.

Figure 5: Scanning electron microscopy of the tail end of A. galli treated with PZQ/PTP. The tail is deflated. A small terminal spine is seen at the tip.
ABZ-treated nematode revealed finer cuticular shrinkage. As shown in Figure 6, the cephalic region with the lips and surrounding cuticle are all shrunken. The cuticular annulations are also evidently contracted. The cuticular folds are much smaller than those observed on PZQ/PTP-treated nematode (Figure 7). The tail end with an anal opening are also severely shrunken (Figure 8).

Figure 6: Scanning electron microscopy of the anterior part of A. galli treated with ABZ. The lips are deflated, shrunken and surrounded by contracted cuticle.

Figure 8: Scanning electron microscopy of the tail end of A. galli treated with ABZ. Deflation is very slight but cuticular folds can be seen. The terminal spine is intact.
Table 1: Efficacy of praziquantel/pyrantel pamoate (PZQ/PTP) and albendazole (ABZ) against the poultry nematode, A. galli.
| Treatment | Dose (mg/ml) | Normalised survival time in hr (± SD) | t value against control | t value between drugs |
| Control | 0 | 100.00 ± 1.21 | - | - |
| PZQ/PTP | 1.25 | 096.73 ± 1.56 | 004.05* | 049.51* |
| 2.5 | 090.44 ± 1.58 | 011.80* | 071.05* | |
| 5 | 086.98 ± 1.09 | 019.62* | 113.10* | |
| 10 | 076.01 ± 1.28 | 033.34* | 094.53* | |
| 20 | 062.28 ± 1.11 | 056.43* | 124.50* | |
| ABZ | 1.25 | 056.27 ± 1.25 | 061.64* | 049.51* |
| 2.5 | 037.90 ± 0.89 | 101.33* | 071.05* | |
| 5 | 025.17 ± 0.78 | 127.57* | 113.10* | |
| 10 | 007.96 ± 1.21 | 131.98* | 094.53* | |
| 20 | 001.77 ± 0.44 | 187.27* | 124.50* |
*Significantly different at p < 0.05; n: 6.
DISCUSSION
Nematodes are the only group of parasites endowed with thick protective body covering, the cuticle. The nematode cuticle is a syncytium of proteins, collagen being the major component. It acts not only as a protective layer but also as the structural framework of the body (Maizels et al., 1993; Decraemer et al., 2003). Thus, it is in a truest sense a durable and anisometric exoskeleton that maintains the rigid contour of the body while allowing flexibility for movement (Basyoni and Rizk, 2016). It is highly resistant to most chemicals including the digestive enzymes of the host. Although, nematodes are the only helminths with proper mouth and alimentary tract, cuticle still serves as the main entry site for anthelmintic drugs. Drugs are known to enter by passive diffusion through the cuticle as in other helminths (Mottier et al., 2006; Lanusse et al., 2016; Abongwa et al., 2017).
In nematodes, it is obvious that anthelmintic drugs interfere with the structural integrity of the cuticle by targeting the cuticular proteins as a primary mode of action (Page et al., 2014). Yet different drugs exhibit different efficacy owing to their differential capacities of diffusion, and no drug is 100% effective on nematodes (Stepek et al., 2006). For instance, ABZ is one of the most lipophilic drugs, and thus one of the most diffusible drugs. It has been shown than ABZ has higher efficacy against Haemonchus contortus and Ascaris suum due to its higher transcuticular diffusion compared to other benzimidazoles (BZDs) or its derivatives (Alvarez et al., 2007).
Our findings also reveal that ABZ directly attacks the cuticle of A. galli. Disintegration of the cuticle is a definitive sign of the anthelmintic effect. In a similar pattern, nitazoxanide acts through the cuticle and was shown to kill the adults and microfilariae of Brugia malayi by destroying the cuticular organisation (Rao et al., 2009). Our study revealed that the extent of damage caused by ABZ on nematode cuticle is quite minimal, implying that the drug acts mostly on the underlying tissues and muscles to cause paralysis and the ensuing death. This supports the fact that ABZ is an established inhibitor of β-tubulin (Alvarez et al., 2007).
We have shown that PTP on the other hand caused extensive cuticular disintegration throughout the body. The cephalic organs are completely shrunk, and the general body is also severely constricted. These indicate that the main target of the drug is the cuticular proteins. However, PTP can also act on the underlying muscle and the intestine as demonstrated in Toxcara canis (Mackenstedt et al., 1993). It is unique in that it can be absorbed both through the cuticle and the alimentary tract via the mouth (Kopp et al., 2008). It is important to note that the cuticular damages seen on A. galli is due to PTP alone, since PZQ is established to be ineffective on nematodes because it is not diffusible through the cuticle. PZQ, with relatively slow uptake through the mouth in the nematodes, enhances the activity of combined drugs by acting synergistically (Southworth et al., 1996; Piantavini et al., 2014). Thus, the vermicidal effects of PTP on A. galli could have been augmented by PZQ by acting on internal tissues other than the cuticle.
CONCLUSION
Albendazole and praziquantel/pyrantel pamoate are commonly used drugs for helminthiases involving infections with cestodes, trematodes, and nematodes. We show here that both drugs are effective against the intestinal nematode, A. galli. For the PZQ/PTP combination drug, the anthelmintic effect on the nematode appeared mostly due to PTP as PZQ is not effective in nematodes. The synergistic effect of PZQ cannot be ruled out, but the drug did not target the cuticle, if at all. ABZ was more effective although it caused less extensive damage on the cuticle of the nematode. This suggests that ABZ mostly act on the sub-cuticular and underlying muscle tissues. Our findings support that the two drugs may be prescribed for helminthiasis due to nematodes in poultry, and that ABZ may be the better drug of choice.
ACKNOWLEDGMENT
The study was funded by the Science and Engineering Research Board, Government on India (EMR/2016/004053). PBL is a Senior Research Fellow under the scheme. SEM was done at the Sophisticated Analytical Instrument Facility (SAIF), North-Eastern Hill University, Shillong, India.
AUTHORS CONTRIBUTION
KLC designed the experiments, analysed the data, and wrote the draft. PBL performed the experiments and finished the manuscript.
CONFLICT OF INTEREST
The authors have declared no conflict of interest.
REFERENCES






