Advances in Animal and Veterinary Sciences
Research Article
Evaluation of the Florfenicol Treatment of Infections Caused by Streptococcus spp and Aeromonas spp in Tambaqui (Colossoma macropomum)
Marcos Ferrante1*, Fernando Marcos Rubim1, Daniela Aparecida de Jesus Paula1, Gilmara Junqueira Machado1, João Pedro Ferreira Guimarães2, Luís David Solis Murgas1
1Section of Physiology and Pharmacology, Department of Veterinary Medicine -DMV. Federal University of Lavras -UFLA, Brazil; 2Zootechnics Department -DZO of Federal University of Lavras –UFLA, Brazil.
Abstract | The aim of this present work is to evaluate the effect of the dose treatment of 21.1 mg/kg oral florfenicol in the treatment of infections caused by Streptococcus spp and Aeromonas spp in tambaqui (Colossoma macropomum). A Monte Carlo simulation of the PK and PD parameters was performed and the PK/PD modelling was carried out to determine the efficacy rates in the treatment of the infections. The efficacy rates obtained were 77% and 73% of the animals with bacteriostatic effect, 16% and 15% of the animals with bactericidal effect and 6% and 10% of the animals with bacteriological eradication for the treatment of Streptococos spp and Aeromonas spp respectively. The present study allowed to establish that the evaluated dose presented a rate of less than 89% of bacteriological eradication for cases of bacterial infections with MICs higher than 0.062 ug / mL. This demonstrates the necessity of incorporating into the therapeutic protocol the bacteriological isolation, the determination of the MIC and the optimization of therapeutic dose according to the bacterial susceptibility in order to avoid therapeutic failures and consequently to promote the development of bacterial resistance.
Keywords | Pharmacokinetic/pharmacodinamic model, Aquiculture, Fish, Antibiotic therapy, Florfenicol.
Received | December 23, 2019; Accepted | March 03, 2020; Published | May 25, 2020
*Correspondence | Marcos Ferrante, Section of Physiology and Pharmacology, Department of Veterinary Medicine -DMV. Federal University of Lavras -UFLA, Brazil; Email: marcos.ferrante@ufla.br
Citation | Ferrante M, Rubim FM, Paula DAJ, Machado GJ, Guimaraes JPF, Murgas LDS (2020). Evaluation of the florfenicol treatment of infections caused by streptococcus spp and aeromonas spp in tambaqui (Colossoma macropomum). Adv. Anim. Vet. Sci. 8(6): 624-628.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.6.624.628
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Ferrante et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
The intensive production system of tambaqui (Colossoma macropomum) has been growing rapidly. Intensive breeding systems, however, can lead to infectious and parasitic disease problems which represent a major concern to fish farming. The ease of disease transmission and the proximity of animals in intensive systems contributes for dissemination. These diseases are considered major problems for fish farming because they can be potential risks to production, with high mortality and loss of profitability (FAO. 2016).
The bacterioses that commonly affect fish farming are those caused by the genus Aeromonas, Pseudomonas, Flavobacterium columnare, Streptococcus spp (Pilarski et al., 2008). The infections caused by Aeromonas ssp are known as hemorrhagic septicemia, which often affect the breeding of freshwater fish (Peixoto et al., 2012). This bacterium is naturally found in the natural gut microbiota of healthy fish and in farmed pond water. However, with poor conditions and low immunity, it manifests itself as a disease (Saharia et al, 2018). While infections by Streptococcus spp in fish are characterized by septicemic, meningoencephalic and abscesses in the muscles of animals, the main clinical signs besides abscesses, are erratic swimming, loss of scales, tegument darkening, anorexia, excitability and death. The disease is endemic in squads and, in cases where the treatment is not performed, it can be spread on the property through successive production cycles (Agnew & Barnes, 2007).
Florfenicol (FF) is a broad spectrum systemic antibiotic specially developed for veterinary use. It is derived from thiamphenicol, which contains a fluorine atom on the third carbon, instead of the hydroxyl group (Dorey et al., 2017). In aquaculture, FF is indicated for the treatment of bacteria diseases caused by Flavobacterium columnare, Aeromonas ssp, and Streptococcosis caused by Streptococcus agalactiae (Bowker et al., 2010).
The manufacturer of florfenicol in fish farming (DSM) indicates doses of 10mg / kg of the drug (Aquaflor®) for tilapias and their hybrids, nonetheless its usage is not indicated in Tambaqui (Gozi, 2016). Castelo Branco (2016) Carried out a pharmacokinetic and depletion study of florfenicol residues after the administration of 10mg / kg in tambaqui. On the other hand, PK parameters were not used to evaluate the efficacy of doses in the treatment of the main bacterial diseases.
The PK / PD models are widely used as indicators of efficiency of human and animal antibiotic treatments (Ema, 2016; Abdelraouf et al., 2017). The utilization of these models along with the Monte Carlo simulation model has been described by Dudley et al. (2000) and Trang et al. (2017). This is currently the main methodology employed for the evaluation and optimization of therapeutic doses of antibiotics (Ema, 2016; Toutain, 2016; Toutain et al., 2017; Rey et al., 2014; Wei et al., 2017).
Regarding the florfenicol, the adequate PK / PD rates are the area below the plasma concentration curve until 24 hours after administration / minimum inhibitory concentration AUC24 / MIC (Ahmad et al., 2016). This value permits to determine if an administrated dose will result in a bacteriostatic or bactericidal effect, or even in bacteriological eradication, considered this last one when the reduction in the number of bacteria is greater than 4 log (Dorey et al., 2017). Thus, the PK / PD models allow the identification of therapeutic dose failures which increase the risk of resistant strains being selected.
The aim of the present study is to evaluate the effect of the treatment with oral doses of 21.1mg/kg of florfenicol in the treatment of infections caused by Streptococcus spp and Aeromonas spp in tambaqui utilizing the PK / PD modelling.
MATERIALS AND METHODS
Research Location
The study was performed at the Department of Veterinary Medicine, Maringá State University, Paraná State, Brazil.
Treatments
The efficacy of the treatment with oral doses of florfenicol 10 mg / kg in the treatment of bacterial infections caused by Streptococcus spp and Aeromonas spp was evaluated. The Efficacy according to the MIC value of the isolated microorganisms, considering the range of 0.016 -2ug / mL was also evaluated.
Pharmacokinetic and Pharmacodynamic Parameters
PK values were obtained from studies conducted in Tambaqui, which presented ABC0-24 values of 9.1 ± 5.1 µg / mL determined after oral administration of 21 mg / kg (Castelo Branco, 2016) (Figure 1). On the other hand, PD parameters were obtained from epidemiological studies on 67 Streptococcus spp isolates and 33 Aeromonas spp isolates in Brazil, with MIC values of 0.61 ± 0.30 μg / mL and MIC of 0.66 ± 0.34 respectively (GOZI, 2016) (Figure 2).

Figure 1: Pharmacokinetics of florfenicol in the doses of 21.1mg / kg orally in Tambaqui (Adapted from Castelo Branco, 2016).
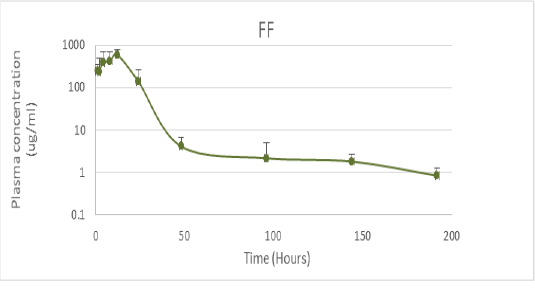
Figure 2: Frequency distribution of the minimum concentration inibitória (MIC) of florfenicol in strains isolated in Brazil. A) Streptococcus spp B) Aeromonas spp. (Adapted from Gozi, 2016).
Pharmacokinetic and Pharmacodynamic Analyzes
A Monte Carlo simulation of the PK and PD parameters of 10,000 individuals was performed, then the PK / PD modeling was performed to determine the efficacy rates of the methodology described by Dudley et al. (2000) and Trang et al. (2017). The effectiveness parameter used was the ABC0-24 / MIC value (Martinez et al., 2007; Ahamad et al., 2016). The ABC24 / MIC values regarding the bacteriostatic and bactericidal effect, along with bacteriological eradication were 0-25; 25-50 and greater than 50, respectively (Dorey et al., 2017).
Statistical Analysis
Statistical analyzes were performed using Excel and BioEstat 5.0 software. For the comparison of the efficacy rates, the chi-square test with a significance of (p <0.05) was used.
RESULTS
Variations in efficacy rates related to MIC variations were observed. The dose with an efficacy rate of 21.1 mg / kg according to MIC value is represented in Figure 3. Eradication rates showed a significant distribution (p <0.05) of efficacy as florfenicol MIC values increased. The eradication rates higher than 90% were found for bacterial infections with MICs ≤ 0.032 μg / mL (Figure 3).
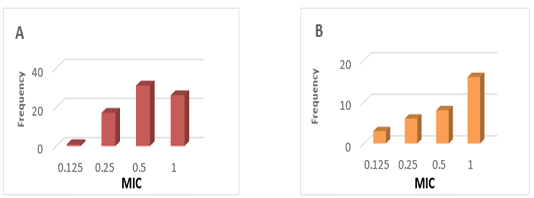
Figure 3: Probability of reaching the index ABC24/MIC according to MIC of the infectious bacterium after oral treatment of 21.1mg / kg of florfenicol in tambaqui. Based on simulation of Monte Carlo (n=10.000).
It has also been observed that the effect of the drug possesses a bacteriostatic action that increases when the MIC concentrations are increased as well, whereas its bactericidal action has a maximum growth limit and subsequently the probability of reaching the ABC24 / MIC index tends to decrease with the increase of MIC rates.
DISCUSSION
The PK / PD model allowed to estimate the efficacy of the oral florfenicol treatment with 21.1 mg / kg according to the bacteria that caused the infection considering the MIC frequency distributions described by Gozi (2016), in his epidemiological studies illustrated in Figure 2. The results of the present study demonstrate low efficacy rates of this dose for the treatment of Streptococcus spp and in tambaqui. Therefore, studies are required to adjust therapeutic doses and achieve adequate bacteriological eradication rates.
The therapeutic success of antibiotic therapy primarily depends on the relation between the drug concentration and site of infection with the minimum inhibitory concentration of the bacteria that is causing the infection (Ahmad et al., 2016; Toutain et al., 2002). Then the therapeutic failure could be from PK or PD origin or a combination of both. The Therapeutic failures originated from PK appear when the drug does not reach the required concentration at the site of action, and from PD origin when the bacteria resists to the original therapeutic concentrations (Hu & Cheng 2016; Torneke & Torren-do, 2015; Toutain et al., 2016). Thus, the choice of therapeutic doses should be based on the MIC of the infection-causing microorganisms for the selected drug (Boothe & Boothe, 2015). By this perspective, the analysis of efficacy according to the MIC establish the complementary information for rational use of antibiotics (Rey et al., 2014; Wei et al., 2017).
The present study allowed to establish that oral florfenicol doses of 21.1 mg / kg achieved a bacteriological eradication rate of less than 89% in cases of bacterial infections, in which the bacteria presented MICs higher than 0.062 μg / mL. (Figure 3). Therefore, in order to use this drug therapeutically on these cases, the corresponding dose adjustments should be made.
Considering also the maximum residue limits for florfenicol in fish edible tissues, the withdrawal period after treatment with 10 mg / kg doses for 10 days is less than 24 hours (Castelo Branco, 2016). The utilization of oral doses of 21.1 mg / kg could provoke higher concentrations of residue limits after 10 days of treatment. However, the realization of the therapeutic doses evidenced in this study also implies the necessity to perform studies regarding the determination of withdrawal periods on the adjusted doses.
In conclusion, the PK / PD design allowed to estimate the subtherapeutic effects of oral doses of 10 mg / kg florfenicol in Tambaqui, demonstrating the necessity of optimization of the therapeutic doses. Furthermore, this study demonstrates that small increases in MIC show large changes in efficacy rates. This highlights the necessity of incorporating therapeutic protocols for bacteriological isolation, MIC determination and therapeutic dose optimization according to the specific susceptibility of bacteria, in order to avoid therapeutic failures and consequently contribute to the inhibition of bacterial resistance development.
ACKNOWLEDGEMENTS
The authors are grateful to the funding agencies that encourage technological and scientific development: CAPES, CNPq and FAPEMIG.
conflict of interest
The authors declared there is no conflict of interest.
authors contribution
All authors contributed equally in the planning of the study, drafting the manuscript. All of them approved the final version of the article.
REFERENCES





