Advances in Animal and Veterinary Sciences
Research Article
Respiratory Affections in Calves in Upper and Middle Egypt: Bacteriologic, Immunologic and Epidemiologic Studies
El-Seedy F.R1, Hassan H.M2, Nabih A.M2, Salem S.E2, Khalifa E3, Menshawy A.M.S4, Abed A.H1*
1Bacteriology, Mycology and Immunology Department, Faculty of Veterinary Medicine, Beni-Suef University, Egypt; 2Animal Reproduction Research Institute, Giza, Egypt; 3Microbiology Department, Faculty of Veterinary Medicine, Matrouh University, Egypt; 4Veterinary Medicine Department (Infectious Diseases), Faculty of Veterinary Medicine, Beni-Suef University, Egypt.
Abstract | Bovine respiratory disease is considered one of the most common and serious problems affecting calves all over the world. The objectives of this study were to perform a bacteriologic, immunologic, and epidemiologic studies on calves suffering from respiratory manifestations (fever, rapid breathing and nasal discharges) in 5 Governorates in Upper and Middle Egypt (Giza, El-Fayoum, Beni-Suef, Assiut and Sohag) with special reference to P. multocida and M. haemolytica as important causes of BRD. Deep nasal swabs and blood samples were collected from 406 bovine calves showing respiratory manifestations. Bacteriologic examination was achieved for isolation of P. multocida, M. haemolytica and other bacteria. The overall prevalence of both P. multocida and M. haemolytica infections was 26.6% (18.2% for P. multocida and 8.4%for M. haemolytica). The highest prevalence was reported in EL-Fayoum Governorate, whereas the lowest prevalence was in Beni-Suef. P. multocida was isolated singly from 4.9% and mixed with other bacteria from 13.3% of the infected calves. On the other hand, M. haemolytica was isolated singly from 1.7% and mixed with other bacteria from 6.7% of the infected calves. Mixed infections of both P. multocida and M. haemolytica with S. aureus were the most prevalent (4% and 2.7%, respectively), followed by mixed infection with both S. aureus and Streptococcus spp. (3.2% and 2.5%, respectively) and finally mixed infection with Streptococcus spp. (2.2% and 1.2%, respectively). More over, 56.2% of the samples showed isolation of other bacteria and 17.2% revealed no bacterial isolates. The in in-vitro sensitivity testing of P. multocida and M. haemolytica isolates showed high susceptibility to fluoroquinolones and cephalosporins. On the contrary, high resistances were obtained against tetracyclines, penicillins and aminoglycosides. Immunologically, all respiratory-manifested calves showed a significant elevation of serum nitric oxide and interleukin-6 levels compared with normal control calves while elucidated significant reduction of lysozyme activity.
Keywords | P. multocida, M. haemolytica, Bovine respiratory disease, Nitric oxide, Lysozyme, IL-6, Antimicrobial susceptibility
Received | December 12, 2019; Accepted | March 31, 2020; Published | May 03, 2020
*Correspondence | Abed, A.H., Bacteriology, Mycology and Immunology Department, Faculty of Veterinary Medicine, Beni-Suef University, Beni-Suef 62511, Egypt; Email: Ahmedabedvet@yahoo.com
Citation | El-Seedy FR, Hassan HM, Nabih AM, Salem SE, Khalifa E, Menshawy AMS, Abed AH (2020). Respiratory affections in calves in upper and middle Egypt: Bacteriologic, immunologic and epidemiologic studies. Adv. Anim. Vet. Sci. 8(5): 558-569.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.5.558.569
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 El-Seedy et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Bovine respiratory disease (BRD) is considered one of the most common worldwide affections in calves causing huge economic losses due to reduction of feed efficiency, average daily gain, overall performance and finally calf mortality (Härtel et al., 2004; Taylor et al., 2010). The incidence of BRD has been reported with variability from 5 to 66% in feedlot cattle and it is the most costly beef cattle disease (Snowder et al., 2006).
BRD in calves is a multi-etiological entity associated with several infectious agents and other factors (Fulton, 2009). Besides infectious agents, multiple environmental and managerial factors and their interactions are responsible for the disease outbreaks (Kabeta et al., 2015).
Most of BRD are of endogenous source caused by the commensal bacteria in the upper respiratory tract (URT). As many factors can impair the local defense mechanisms and/or damage the lining of the respiratory tract to such an extent that these pathogens are able to progress deeper into the respiratory tract and cause disease (Lopez, 2001). Any one or a combination of the environmental and managerial factors can make calves more susceptible to the disease. Also, exogenous infections can occur by direct contact with diseased animals or infected aerosols.
Acute pneumonia in calves is associated with cough, sneezing, mucoid to mucopurulent nasal discharge, congested nasal mucous membrane, elevated pulse and respiratory rates as well as fever and depression (Yehia, 2000; El-Sebaieet al., 2002).
Bacterial infections causing pneumonia in calves can possibly be fatal. The infection is usually a sum of three codependent factors including stress, an underlying viral infection, and a new bacterial infection (Fulton and Confer, 2012). Under unsuitable conditions, viruses induce immunosuppression and damage to the respiratory epithelia that may lead to secondary bacterial respiratory infection and clinical signs of BRD (Ellis, 2009).
Pasteurella multocida (P. multocida) and Mannheimia haemolytica (M. haemolytica) are the most two common bacterial causes of calves pneumonia. Numerous other bacteria can also cause pneumonia including Mycoplasma, Pseudomonas aeruginosa, Corynebacteria (especially C. bovis), Staphylococcus spp., Haemophilus, E. coli, Streptococcus spp., Proteus spp., Actinomyces pyogenes, Klebsiella pneumoniae, Bordetella, Neisseria, Erysipelothrix and Fusobacterium (Sayed and Zaitoun, 2009; Asaye et al., 2015). Pneumonic pasteurellosis refers to any disease conditions caused by bacteria of the genera Pasteurella or Mannheimia especially P. multocida and M. haemolytica which are the most commonly associated bacteria with calf pneumonia (Adamu and Ameh, 2007; Asaye et al., 2015). The disease is manifested mostly in calves within 4 weeks of weaning, as the calves are sorted and distributedto different farms. It is given a common surname “Shipping Fever” (Snowder et al., 2006).
Lysozyme is a small mucosalsecretion enzyme which is naturally found in body secretions such as tears, saliva, and milk. It is apart of the nonspecific opsonin response associated with defense against bacteria (Oien and Moskovitz, 2007). It acts as an antimicrobial agent by cleaving the peptidoglycan component of bacterial cell walls, which leads to cell death (Oliver and Wells, 2015).
Nitric oxide (NO) radicals are produced by leukocytes (Rodenas et al., 2000). It acts as intracellular signaling molecule or as neurotransmitter. Also, when it was produced both in low and in high quantities for extended periods, it kills microorganisms and tumor cells. Nitric oxide was identified as the effect or molecule in killing a wide range intra and extra cellular pathogens (Schmidt and Walter, 1994). Nitric oxide releasing solution (NORS) has potential in the prevention of BRD (Edwards, 2010). It protects against the development of BRD by limiting harmful inflammatory effects while simultaneously increasing and enhancing the ability of the host to detect and respond to bacterial pathogens (Sheridan et al., 2016).
Interleukins have essential roles in key functions of the immune system, tolerance and immunity, primarily via its direct effects on T cells (Liao et al., 2011). The activated macrophages produce important cytokines such as Interleukin-6 (IL-6) contributing to host defense by inducing fever and termed endogenous pyrogens. Expression of cytokines such as IL-6 has a consistent up regulation in pneumonic cattle. They can participate in the immune and inflammatory responses during the pulmonary defense mechanisms (Rodríguez et al., 2015). IL-6 activates endothelial cells, epithelial cells, alveolar macrophages and lung dendritic cells leading to release of chemokines attracting neutrophils and monocytes into the affected area and with time also attracts T and B cells (Ackermann, 2010).
The present study was undertaken to investigate calves with respiratory manifestations in Upper and Middle Egypt; bacteriologically, immunologically, and epidemiologically, with special reference to P. multocida and M. haemolytica infections.
Materials and Methods
Animals
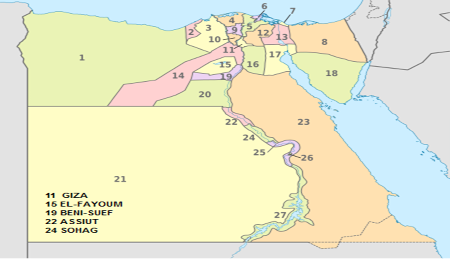
Four hundreds and six pneumonic bovine calves; of both sexes and of different ages,reared in different Upper and Middle Egypt Governorates (Giza, EL-Fayoum, Beni-Suef, Assiut and Sohag) were examined during the period from January 2017 till December 2017 (Table 1). All animals were showing respiratory manifestations such as fever, rapid breathing and nasal discharges.
Samples
A total number of 406 deep nasal swabs were collected under aseptic conditions for bacteriologic examination from calves with respiratory manifestations. Also, blood samples were collected from each calf for bacteriologic and immunologic investigations. Samples were transferred as soon as possible in ice box to bacteriology lab in Animal Reproduction Research Institute (ARRI) in Giza Governorate (AL-Haram).
Table 1: Number of animals and samples collected from respiratory affected bovine calves from different Governorates of Egypt.
| Governorates | Number of affected animals | Number of collected samples | |
| Nasal swab | Blood samples | ||
| Giza | 69(17.0%) | 69 | 69 |
| El-Fayoum | 106(26.2%) | 106 | 106 |
| Beni-Suef | 83(20.4%) | 83 | 83 |
| Assiut | 87(21.4%) | 87 | 87 |
| Sohag | 61(15.0%) | 61 | 61 |
| Total | 406 | 406 | 406 |
Bacteriologic examination
Isolations of P. multocida, M. haemolytica and other bacteria were done according to Collee et al. (1996) and Quinn et al. (2002). The collected nasal swabs were inoculated under aseptic conditions into brain heart infusion broth (BHIB) and incubated aerobically at 37oC for 6-8 hrs. A loopful from broth was cultured onto blood agar, MacConkey’s agar and DAS media then incubated aerobically at 37oC for 24 hrs. Cultivation of other bacteria were achieved using nutrient agar, MacConkey’s agar, blood agar; eosin methylene blue media (EMB), mannitol salt agar; Baird Parker agar and modified Edward’s media then incubated aerobically at 37oC for 24-48 hrs. All the recovered isolates were identified morphologically using Gram’s stain. Moreover, 2 bloodfilms were freshly prepared from each examined calf and stained with Leishman’s stain for detection of Pasteurella bipolarity.
Biochemical identification of the bacterial isolates
All the recovered isolates were identified biochemically according to schemes described by Kreig and Holt (1984), Collee et al. (1996) and Quinn et al. (2002). The suspected isolates of P. multocida and M. haemolytica were tested for haemolysis on blood agar and growth on MacConkey’s agar as well as biochemical tests; oxidase, catalase, indole, triple sugar iron agarmedium, citrate utilization and sugar fermentation (glucose, lactose, sucrose and mannitol) tests.
Antimicrobial susceptibility testing of p. multocida and m. haemolytica.
The in-vitro susceptibility testing was conducted on 20 isolates of both P. multocida and M. haemolytica of which 10 isolates were from single infections and 10 from mixed infections. P. multocida and M. haemolytica isolates were tested for their antimicrobial susceptibility to 12 different antimicrobial discs including amoxicillin-calvulanic acid (30 µg), ampicillin-sulbactam (20 µg), cefotriaxone (30 µg), cefiquinome (30 µg), kanamycin (30 µg), amikacin (30 µg), sulphamethoxazol-trimethoprim (25 µg), ciprofloxacin (5 µg), levofloxacin (5 µg), enrofloxacin (5 µg), oxytetracycline (30 µg) and chloramphenicol (30 µg). Antimicrobial susceptibility testing was performed using disc diffusion method on Muller Hinton agar according to Clinical and Laboratory Standards Institute (CLSI, 2014). The antibiotic susceptibility was based on the induced inhibition zones according to the guidelines of the CLSI (2014).
Immunologic studies
Detection of lysozyme concentration
Assaying for lysozyme was done according to Schltz (1987). The clear zone ring diameters were measured to the nearest 0.1 mm with an enlarger-viewer (Kalesttad Laboratories, Inc., Austin, TX). For each lysoplate, the lysozyme concentrations in the samples were determined from a plotted standard curve against the corresponding clear zone ring diameter on linear axis.
Measurement of serum nitric oxide
The measurement of nitric oxide was assessed according to the assay described by to Rajaramanet al. (1998).
Measurement of Bovine Interleukin-6.
The measurement of IL-6 concentrationwas assessed using ELISA Kit.
Results
Prevalence of p. multocida and m. haemolytica in different Governorates.
The overall prevalence of both P. multocida and M. haemolytica was 108/406 with a percentage of 26.6%. The prevalence of P. multocidawas 18.2% (74/406) while M. haemolytica was 8.4% (34/406) (Table 2).
Prevalence of single and mixed p. multocida infections in different governorates
P. multocida was singly isolated from 20 cases (4.9%) while it was mixed in 54 cases (13.3%). In mixed infection, it was isolated with S. aureus from 16 infected calves (4%). Also, it was mixed with E. coli, Streptococcus spp., both S. aureus and E. coli, both S. aureus and Streptococcus spp. and both E. coli and Streptococcus spp. at rates of 1.2%, 2.2%, 1.7%, 3.2%, and 1.0%, respectively (Table 3).
Table 2: Prevalences of P.multocida and M. haemolytica infections in different Governorates.
| Governorates | No. of samples | P. multocida | M. haemolytica | Total | |||
| No. | % | No. | % | No. | % | ||
| Giza | 69 | 13 | 18.8 | 6 | 8.7 | 19 | 27.5 |
| El-Fayoum | 106 | 22 | 20.8 | 10 | 9.4 | 32 | 30.2 |
| Beni-Suef | 83 | 11 | 13.3 | 5 | 6.0 | 16 | 19.3 |
| Assiut | 87 | 16 | 18.4 | 8 | 9.2 | 24 | 27.6 |
| Sohag | 61 | 12 | 19.7 | 5 | 8.2 | 17 | 27.9 |
| Total | 406 | 74 | 18.2 | 34 | 8.4 | 108 | 26.6 |
%: Percentages were calculated according to the corresponding No. of samples.
Prevalence of single and mixed m. haemolytica infections in different governorates
M. haemolytica was isolated as a single isolate from 7 cases (1.7%) while it was mixed in 27 cases (6.7%). No mixed infection with both E. coli and S. aureus was reported in all Governorates. Totally, it was mixed with S. aureus, Streptococcus spp., both S. aureus and Streptococcus spp. and both E. coli and Streptococcus spp. at rates of 2.7%, 1.2%, 2.5% and, 0.2%, respectively (Table 4).
Prevalence of other bacteria from diseased calves in different governorates
Out of the 406 samples, 228 samples (56.2%) showed bacterial isolation other than P. multocida and M. haemolytica. Meanwhile 70 samples (17.2%) showed no bacterial isolation. The bacterial isolates were identified as 51 S. aureus (12.6%), 41 Streptococcus spp. (10%), 17 E. coli (4.2%), 46mixed S. aureus+ Streptococcus spp. (11.3%), 21 mixed S. aureus E. coli (5.2%), 13 mixed Streptococcus spp. E. coli (3.2%), mixed 17 S. aureus Streptococcus spp. E. coli (4.2%), 11 P. aeruginosa (2.7%), 5 Corynebacterium spp. (1.2%) and 6 Proteus spp. (1.5%). The highest percentages of bacterial isolation were recorded in Beni-Suef, Assiut followed by Giza and El-Fayoum; as 53 (63.9%), 53 (60.9 %), 35 (50.7%), and 60 (56.6%), respectively. Meanwhile the lowest prevalence was recorded in Sohag; 27 (44.3%) (Table 5).
Data illustrated in Table 6 showed the collective prevalences of different bacterial isolates recovered from diseased calves in different Governorates. Out of the 406 samples, 336 samples (82.8%) showed bacterial isolation. Meanwhile 70 samples (17.2%) showed no bacterial isolation.
Antimicrobial susceptibility testing of p. multocida and m. haemolytica isolates
The in-vitro sensitivity testing of P. multocida isolates either single or mixed infections showed high susceptibility to cefiquinome, levofloxacin and ciprofloxacin. On the other hand, they were highly resistant to oxytetracycline, ampicillin-sulbactam, amoxicillin-calvulanic acid and kanamycin (Table 7).
The in-vitrosensitivity testing of M. haemolytica isolates; either single infection or mixed infections, revealed high sensitivity to enrofloxacin, cefiquinome, levofloxacin and ciprofloxacin. On the contrary, they were highly resistant to oxytetracycline kanamycin, amikacin and amoxicillin-calvulanic acid (Table 7).
Results of immunologicparameters
Immune parameters related to p. multocida isolates in respiratory manifested calves
Immune parameters related to single and mixed P. multocida isolates recorded in Table 8 showed that all respiratory manifested calves recorded a significant elevation of serum nitric oxide andIL-6levels compared with normal control calves.
The infected calves with P. multocida, P. multocida+ S. aureus, P. multocida+ E. coli recorded no significant changes (slightly increase) in serum lysozymes. Meanwhile, the infected calves with P. multocida+ Streptococcus spp., P. multocida+ S. aureus+ E. coli, P. multocida+ S. aureus+ Streptococcus spp. and P. multocida+ E. coli+ Streptococcus spp. elucidated asignificant reduction of lysozymes.
Immuneparameters related to m. haemolytica isolates in respiratory manifested calves
Immune parameters related to single and mixed M. haemolytica isolates recorded in Table 9 showed that all respiratory manifested calves recorded asignificant elevation of serum nitric oxide IL-6 levels. However, all calves elucidated a significant reduction of lysozyme activity.
Immune parameters related to other bacterial isolates in respiratory manifested calves
Immune parameters related to single or mixed bacterial isolates other than P. multocida and M. haemolytica illustrated in Table 10 displayed that all calves infected with showed a significant elevation of serum nitric oxide level and IL-6. On the other hand, all the calves elucidated a significant reduction of lysozyme activity.
Discussion
Bovine respiratory disease is a multi-factorial disease, usually resulting from the interaction of bacterial and viral agents,
Table 3: Prevalence of single and mixed P. multocida infections in different governorates.
|
Governorates Isolates |
Giza (No.= 69) |
El-Fayoum (No.= 106) |
Beni-Suef (No.= 83) |
Assiut (No.= 87) |
Sohag (No.= 61) |
Total (No.= 406) |
|||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | ||
|
P. multocida (Single isolate) |
4 | 5.8 | 6 | 5.7 | 3 | 3.6 | 5 | 5.7 | 2 | 3.3 | 20 | 4.9 | |
| Mixed isolates | P. multocida+ S. aureus | 3 | 4.4 | 5 | 4.7 | 3 | 3.6 | 3 | 3.5 | 2 | 3.3 | 16 | 4.0 |
| P. multocida+ E. coli | 1 | 1.4 | 2 | 1.9 | 0 | 0 | 1 | 1.1 | 1 | 1.6 | 5 | 1.2 | |
| P. multocida+ Streptococci. | 1 | 1.4 | 3 | 2.8 | 1 | 1.2 | 2 | 2.3 | 2 | 3.3 | 9 | 2.2 | |
| P. multocida+ S. aureus+ E. coli | 2 | 2.9 | 2 | 1.9 | 1 | 1.2 | 1 | 1.1 | 1 | 1.6 | 7 | 1.7 | |
| P. multocida+ S. aureus+ Streptococci | 2 | 2.9 | 3 | 2.8 | 2 | 2.4 | 3 | 3.5 | 3 | 4.9 | 13 | 3.2 | |
| P. multocida+ E. coli + Streptococci | 0 | 0 | 1 | 0.9 | 1 | 1.2 | 1 | 1.1 | 1 | 1.6 | 4 | 1.0 | |
| Total mixed isolates | 9 | 13 | 16 | 15.1 | 8 | 9.7 | 11 | 12.6 | 10 | 16.4 | 54 | 13.3 | |
| Overall total | 13 | 18.8 | 22 | 20.8 | 11 | 13.3 | 16 | 18.4 | 12 | 19.7 | 74 | 18.2 | |
%: Percentages were calculated according to the corresponding No. of samples in each governorate.
Table 4: Prevalence of single and mixed M. haemolytica infections in different governorates.
|
Governorates Isolates |
Giza (No.= 69) |
El-Fayoum (No.= 106) |
Beni-Suef (No.= 83) |
Assiut (No.= 87) |
Sohag (No.= 61) |
Total (No.= 406) |
|||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | ||
|
M. haemolytica (Single isolate) |
1 | 1.4 | 3 | 2.8 | 1 | 1.2 | 2 | 2.3 | 0 | 0 | 7 | 1.7 | |
| Mixed isolates | M. haemolytica + S. aureus | 2 | 2.9 | 3 | 2.8 | 1 | 1.2 | 3 | 3.5 | 2 | 3.3 | 11 | 2.7 |
| M. haemolytica + Streptococci. | 1 | 1.4 | 1 | 0.9 | 1 | 1.2 | 1 | 1.1 | 1 | 1.6 | 5 | 1.2 | |
| M. haemolytica + S. aureus+ Streptococci | 2 | 2.9 | 2 | 1.9 | 2 | 2.4 | 2 | 2.3 | 2 | 3.3 | 10 | 2.5 | |
| M. haemolytica + E. coli + Streptococci | 0 | 0 | 1 | 0.9 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0.2 | |
| Total mixed isolates | 5 | 7.2 | 7 | 6.6 | 4 | 4.8 | 6 | 6.9 | 5 | 8.2 | 27 | 6.7 | |
| Overall total | 6 | 8.7 | 10 | 9.4 | 5 | 6.0 | 8 | 9.2 | 5 | 8.2 | 34 | 8.4 | |
%: Percentages were calculated according to the corresponding No. of samples in each Governorate.
Table 5: Prevalence of other bacterial isolates from diseased calves in different governorates.
|
Governorates Isolates |
Giza (No.= 69) |
El-Fayoum (No.= 106) |
Beni-Suef (No.= 83) |
Assiut (No.= 87) |
Sohag (No.= 61) |
Total (No.= 406) |
||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| S. aureus | 7 | 10.1 | 12 | 11.3 | 13 | 15.7 | 12 | 13.8 | 7 | 11.5 | 51 | 12.6 |
| Streptococci | 5 | 7.3 | 9 | 8.5 | 10 | 12.0 | 11 | 12.6 | 6 | 9.8 | 41 | 10.0 |
| E. coli | 3 | 4.3 | 5 | 4.7 | 3 | 3.6 | 4 | 4.6 | 2 | 3.3 | 17 | 4.2 |
| S. aureus+ Streptococci | 8 | 11.6 | 12 | 11.3 | 9 | 10.8 | 12 | 13.8 | 5 | 8.2 | 46 | 11.3 |
| S. aureus + E. coli | 3 | 4.3 | 6 | 5.7 | 5 | 6.0 | 5 | 5.7 | 2 | 3.3 | 21 | 5.2 |
| Streptococci +E. coli | 3 | 4.3 | 5 | 4.7 | 2 | 2.4 | 2 | 2.3 | 1 | 1.6 | 13 | 3.2 |
| S. aureus+ E. coli + Streptococci | 2 | 2.9 | 5 | 4.7 | 4 | 4.8 | 4 | 4.1 | 2 | 3.3 | 17 | 4.2 |
| P.aeruginosa | 2 | 2.9 | 3 | 2.8 | 3 | 3.6 | 2 | 2.3 | 1 | 1.6 | 11 | 2.7 |
|
Corynebacterium spp |
1 | 1.4 | 1 | 0.9 | 2 | 2.4 | 1 | 1.1 | 0 | 0 | 5 | 1.2 |
|
Proteus spp. |
1 | 1.4 | 2 | 1.9 | 2 | 2.4 | 0 | 0 | 1 | 1.6 | 6 | 1.5 |
| Total isolates | 35 | 50.7 | 60 | 56.6 | 53 | 63.9 | 53 | 60.9 | 27 | 44.3 | 228 | 56.2 |
| No bacterial growth | 15 | 21.7 | 14 | 13.2 | 14 | 16.9 | 10 | 11.5 | 17 | 27.9 | 70 | 17.2 |
| Overall Total | 50 | 72.5 | 74 | 69.8 | 67 | 80.7 | 63 | 72.4 | 44 | 72.1 | 298 | 73.4 |
%: Percentages were calculated according to the corresponding No. of samples in each Governorate.
combined with stress factors such as transportation, weather extremes, weaning, dusty conditions, collecting of cattle from multiple sources, parasitic infestation, concurrent diseases and environmental factors (Ellis, 2009; Kabeta et al., 2015). One of the challenges of bovine respiratory medicine is early detection of clinical cases of BRD.
Many of the infectious agents commonly involved in calf pneumonia are commensals in the nasal passages of healthy animals. Many factors can impair the local defense mechanisms and/or damage the lining of the respiratory tract to such an extent that these pathogens are able to progress deeper into the respiratory tract and cause disease (Lopez, 2001). Stress factors such as Overfeeding, overpopulation, cold temperature, bad hygiene, stress and colostrum deprivation, could suppress the mucociliary clearance mechanism which allows the proliferation of bacterial commensals in the respiratory tract that causes an abrupt shift from commensal to pathogen especially in M. haemolytica. This shift has made M. haemolytica to assume greater prominence in bovine pneumonia (Griffin et al., 2010). Any one or a combination of the environmental and managerial factors can make calves more susceptible to disease.
This is especially important in subclinical forms of the disease, which can be easily missed and cause important economic losses. It is generally accepted that early recognition and treatment of BRD improves both prognosis and outcome, while delayed diagnosis and treatment may result in treatment failure.
Numerous bacteria such as Mycoplasma, E. coli, Staphylococcus spp., Streptococcus spp., P. aeruginosa, Proteus spp., Corynebacteria and, K. pneumoniae can cause pneumonia (Sayed and Zaitoun, 2009; Asaye et al., 2015). However, P. multocida and M. haemolytica remain the most common bacterial pathogens associated with pneumonia in cattle calves (Asaye et al., 2015).
In the present study, the prevalence of P. multocida and M. haemolytica in different Governorates were investigated and presented in Table 2. The results showed that the overall prevalence of both P. multocida and M. haemolytica was 26.6% (18.2% and 8.4% for P. multocida and M. haemolytica, respectively). These results were nearly similar to those reported in Egypt by Zaki et al. (2002) who isolated P. multocida in a high prevalence rate (19.9%) in comparison to P. haemolytica (8.8%) from 226 lung sample taken from pneumonic calves aging 1 day to 2 month old in Kaliobia and Sharkia Governorates. A higher prevalence of P. multocida was obtained in Egypt by El-Jakee et al. (2016) who recovered 88 P. multocida isolates (34.4%) from 256 nasopharyngeal swabs and lung tissues samples from different Egyptian Governorates. It was found that dead calves showed the highest percentage of P. multocida isolation followed by the emergency slaughtered calves, diseased calves then apparently healthy ones. Also, Ahmed et al. (2015) estimated the prevalence of Pasteurella spp. in upper respiratory tract of cattle. Pasteurella spp. was recovered at a rate of 38.9%. P. multocida and M. haemolytica were recovered from the samples at a rate of 30% 20% respectively.
In the same context, Abera et al. (2014); in Western Ethiopia, found that the overall percentage of P. multocida was 39.3% and M. haemolytica was 46.4%; results that were higher than those reported in the current study. This may possibly be due to the fact that the etiology of pneumonia is a complex and multifactorial. On the other hand, lower prevalence was recorded in Nigeria by Lasisi et al. (2016) who isolated P. multocida and M. haemolytica from 6 (7.22%) and 1 (1.22%) unhealthy lung tissue sample of cattle, respectively.
Regarding distribution of infection, EL-Fayoum Governorate showed the highest overall prevalence (30.2%), whereas Beni-Suef one showed the lowest rate of infection (19.3%). P. multocida and M. haemolytica could be isolated at higher percentages (20.8 and 9.4%, respectively) in EL-Fayoum Governorate, whereas the lowest prevalence concerning these bacteria was in Beni-Suef (13.3 and 6.0%, respectively). These findings might be attributed to the different hygienic measures and stress factors in different Governorates. The highest prevalence that recorded in El-Fayoum because that governorate has intensive calves rearing systems, the young suckling calves are collected from different sources or localities to be reared together in closed farms and that usually puts young calves under stressful conditions leading to decreased immunity and later on increases susceptibility for infectious diseases especially respiratory pathogens. Beni-Suef had a lower prevalence rate due to the lower intensive calf rearing and production systems.
Prevalences of single and mixed P. multocida and M. haemolytica infections in the different locations were illustrated in Tables 3 and 4. Totally, P. multocida was isolated singly and mixed from 4.9% and13.3% of the infected calves, respectively. Meanwhile, M. haemolytica was isolated singly and mixed at rates of 1.7% and 6.7%, respectively. Regarding mixed infections, P. multocida and M. haemolytica were isolated in combination with S. aureus at rates of 4 and 2.7%, respectively and with both S. aureus plus Streptococcus spp. at a prevalence levels of 3.2 and 2.5%, respectively and finally with Streptococcus spp. with 2.2 and 1.2 %, respectively. The current findings might be due to presence of S. aureus and Streptococcus spp. as a normal flora on the skin and oropharynx which may be flourished and causing diseases as a result of bad hygienic measures or environmental and managerial stresses or any factor that could decrease the host’s immune system and/or damage the lining of the respiratory tract to such an extent that these pathogens are able to progress deeper into the respiratory tract and cause disease (Kabeta et al., 2015; Lopez, 2001).
Table 5 showed that 56.2% of the calf samples were positive for bacteria other than P. multocida and M. haemolytica. These results were coincided with those recorded by Singh and Singh (1980); in India, Munoz et al. (1984) and Simko and Lehocky (1993) who revealed that S. aureus, Streptococcus spp. and E. coli were the most prevalent bacterial isolates from calves with respiratory manifestations. Mansour (1997); in Egypt, found that S. aureus, Streptococcus spp. were the most prevalent isolates from dead calves having septicemia and respiratory manifestation. Moreover, Hafez and Yousef (2002) and Sayed et al. (2002) in Egypt, recorded that beside P. multocida and M. haemolytica, S. aureus and E. coli were the most prevalent bacteria.
Collectively, the current results were similar to those recorded in Egypt by Sedeek and Thabet (2001) who studied 82 samples from pneumonic cattle at Assiut Governorate. Ninety two isolates were recovered of which 18 S. aureus (25%), 8 S. pyognes (11%), 8 P. aeruginosa (11%),7 E. coli (9.7%),6 S. pneumoniae (8.3%), 6 P. multocida (8.3%) and 2 Proteus vulgaris (2.8%). Also, Sayed and Zaitoun (2009) found that the most prevalent bacterial isolates from 66 lungs from calves having respiratory diseases were S. aureus (22.4%) and E. coli (18.2%). Proteus vulgaris, S. pyogenes, and Corynebacterium bovis were also recovered as 7.0%, 5.6% and 2.8%, respectively.
Data illustrated in Table 6 showed the collective prevalence of different bacteria isolated from diseased calves in different Governorates. Out of the 406 samples, 336 samples (82.8%) showed bacterial isolation. Meanwhile 70 samples (17.2%) showed no bacterial isolation.
The negative bacterial isolation was not necessarily meaning the absence of bacterial infections. It may be attributed to the fact that some microorganisms can’t grow on the used culture media as they require specific/enriched culture media or tissue culture including Mycoplasma spp.; especially M. bovis as well as Histophilus somnus (Confer, 2009; Maunsell et al., 2011; Kabeta et al., 2015) and chlamydia (Register et al., 2015); which are all mostly incriminated in BRD affecting young calves.
P. multocida and M. haemolyticainfections are commonly managed by broad spectrum antimicrobials (Kehrenberg et al., 2001). Therefore, investigation of their antimicrobial susceptibility trends is considered an important aid (Katsuda et al., 2013b). The results of in-vitro antimicrobial susceptibility tests of P. multocida and M. haemolytica isolates were illustrated in Table 3. The in-vitro sensitivity testing of P. multocida isolates showed high susceptibility to cefiquinome, levofloxacin and ciprofloxacin. Meanwhile, high resistances were obtained against oxytetracycline, ampicillin-sulbactam, amoxicillin-calvulanic acid and kanamycin.
Table 6: Collective prevalences of different bacterial isolates from diseased calves.
| Bacterial Isolates | Total (No.= 406) | |
| No. | % | |
|
Single P. multocida isolate |
20 | 4.9 |
|
Mixed P. multocida isolates |
54 | 13.3 |
|
Total P. multocida isolates |
74 | 18.2 |
|
Single M. haemolytica isolate |
7 | 1.7 |
|
Mixed M. haemolytica isolates |
27 | 6.7 |
|
Total M. haemolytica isolates |
34 | 8.4 |
| S. aureus | 51 | 12.6 |
| Streptococci | 41 | 10.0 |
| E. coli | 17 | 4.2 |
|
S. aureus+ Streptococci |
46 | 11.3 |
| S. aureus + E. coli | 21 | 5.2 |
| Streptococci +E. coli | 13 | 3.2 |
|
S. aureus+ E. coli + Streptococci |
17 | 4.2 |
| P. aeruginosa | 11 | 2.7 |
|
Corynebacterium spp. |
5 | 1.2 |
|
Proteus spp. |
6 | 1.5 |
| Total other isolates | 228 | 56.2 |
| Total bacterial isolates | 336 | 82.8 |
| No bacterial growth | 70 | 17.2 |
| Total No. of samples | 406 | 100 |
%: Percentages were calculated according to the total No. of samples.
Regarding M. haemolytica, singleisolate was highly sensitive to enrofloxacin, cefiquinome, levofloxacin, ciprofloxacin and ceftriaxone in a percentage of 90, 80, 80, 70 and 70%, respectively. Meanwhile, high resistances were showed against oxytetracycline, kanamycin, amikacin and amoxicillin-calvulanic acid, in a percentage of 100, 90, 70 and 60%, respectively. In case of mixed infections, the isolates were highly sensitive to cefiquinome (80%) and 70% for each of levofloxacin, ciprofloxacin and enrofloxacin. On the other hand, complete resistances were shown against oxytetracycline and kanamycin (100% for each). Meanwhile amoxicillin/calvulanic acid and Amikacin were resistance in a rate of 80% for each.
Generally, the in-vitro sensitivity testing of the isolates showed high susceptibility to fluoroquinolones and cephalosporins. On the other hand, high resistances were obtained against tetracyclines, penicillins and aminoglycosides. The obtained data were agreed with the Katsuda et al. (2013a); in Japan, who recorded high resistance of P. multocida to oxytetracycline while ceftiofur, cefquinome and enrofloxacin were highly effective. Also, it was agreed with Hendriksen et al. (2008); in different European countries, who observed that all isolates of
Table 7: Antibacterial sensitivity testing of single and mixed P. multocida and M. haemolyticaisolates.
| Antimicrobial Discs | Discs Conc. (µg) |
P. multocida isolates |
M. haemolytica isolates |
||||||
| Single isolates | mixed isolates | Single isolates | mixed isolates | ||||||
| Sensitive | Resistant | Sensitive | Resistant | Sensitive | Resistant | Sensitive | Resistant | ||
| Amoxicillin-calvulanic acid | 30 | 30% | 70% | 20% | 80% | 40% | 60% | 20% | 80 |
| Ampicillin-sulbactam | 20 | 20% | 80% | 10% | 90% | 50% | 50% | 30% | 70 |
| Cefiquinome | 30 | 90% | 10% | 80% | 20% | 80% | 20% | 80% | 20 |
| Cefotriaxone | 30 | 60% | 40% | 30% | 70% | 70% | 30% | 50% | 50 |
| Amikacin | 30 | 60% | 40% | 40% | 60% | 30% | 70% | 20% | 80 |
| Kanamycin | 30 | 30% | 70% | 20% | 80% | 10% | 90% | 0.0% | 100 |
| Sulphamethoxazol-trimethoprim | 25 | 70% | 30% | 50% | 50% | 40% | 60% | 40% | 60 |
| Levofloxacin | 5 | 90% | 10% | 80% | 20% | 80% | 20% | 70% | 30 |
| Ciprofloxacin | 5 | 90% | 10% | 70% | 30% | 70% | 30% | 70% | 30 |
| Oxytetracycline | 30 | 10% | 90% | 0.0% | 100% | 0.0% | 100% | 0.0% | 100 |
| Enrofloxacin | 5 | 70% | 30% | 60% | 40% | 90% | 10% | 70% | 30 |
| Chloramphenicol | 30 | 50% | 50% | 30% | 70% | 40% | 60% | 30% | 70 |
Table 8: Immune parameters related to P. multocida isolates causing calves respiratory infection.
| Bacterial isolates |
Immune parameters |
||
| Nitric oxide | Lysozymes | Interleukin 6 | |
|
P. multocida (single isolate) |
9.29 ± 1.32 | 14.13 ± 0.95 | 143.6 ± 15.6 |
| P. multocida+ S. aureus | 10.15 ± 0.82 | 12.66 ± 1.05 | 129.7 ± 10.33 |
| P. multocida+ E. coli | 8.05 ± 0.91 | 12.15 ± 1.16 | 110.4 ± 8.351 |
|
P. multocida+ Streptococci |
11.33 ± 0.52 | 8.33 ± 0.13 | 136.2 ± 11.83 |
| P. multocida+ S. aureus+ E. coli | 14.51 ± 1.03 | 7.39 ± 1.01 | 147.5 ± 12.62 |
|
P. multocida+ S. aureus+ Streptococci |
19.65 ± 0.93 | 7.61 ± 0.83 | 159.9 ± 11.73 |
|
P. multocida+ E. coli+ Streptococci |
14.33 ± 0.0 | 7.3 5± o.57 | 140.7 ± 14.36 |
| Normal | 6.35 ± 0.29 | 11.66 ± 1.13 | 75.4 ± 7.38 |
Table 9: Immune parameters related to M. haemolytica isolates causing calves respiratory infection.
| Bacterial isolates |
Immune parameters |
||
| Nitric oxide | Lysozymes | Interleukin 6 | |
|
M. haemolytica (single isolate) |
12.36 ± 1.39 | 7.05 ± 1.32 | 156.2 ± 10.39 |
| M. haemolytica+ S. aureus | 14.62 ± 0.83 | 7.23 ± o.92 | 173.26 ± 12.05 |
| M. haemolytica+ E. coli | 12.03 ± 1.25 | 7.35 ± 0.84 | 123.74 ± 14.83 |
|
M. haemolytica+ Streptococci |
14.66 ± 0.73 | 6.32 ± 0.88 | 139.61 ± 11.56 |
| M. haemolytica+ S. aureus+ E. coli | 16.3 ± 1.33 | 5.11 ± 0.56 | 130.55 ± 12.38 |
|
M. haemolytica+ S. aureus+ Streptococci |
15.46 ± 0.96 | 4.62 ± 0.39 | 158.72 ± 7.66 |
|
M. haemolytica+ E. coli+ Streptococci |
17.05 ± 0.72 | 5.02 ± 0.66 | 153.08 ± 10.54 |
| Normal | 6.35 ± 0.29 | 11.66 ± 1.13 | 75.4 ± 7.38 |
Table 10: Immune parameters related to other bacterial isolates causing calves respiratory infection.
| Bacterial isolates |
Immune parameters |
||
| Nitric oxide | Lysozymes | Interleukin 6 | |
| S. aureus | 8.32 ± 0.92 | 8.15 ± 0.78 | 101.66 ± 3.16 |
| Streptococci | 8.78 ± 0.71 | 8.33 ± 0.92 | 122.5 ± 6.39 |
| E. coli | 7.25 ± 1.31 | 10.36 ± 1.13 | 98.1 ± 5.24 |
|
S. aureus+ Streptococci |
9.33 ± 1.52 | 7.17 ± 0.59 | 122.6 ± 10.52 |
| S. aureus + E. coli | 9.75 ± 0.98 | 9.32 ± 1.32 | 126.7 ± 8.71 |
|
Streptococci +E. coli |
9.01 ± 1.32 | 9.59 ± 1.26 | 122.65 ± 8.36 |
|
S. aureus+ Streptococci + E. coli |
11.33 ± 1.62 | 8.32 ± 0.58 | 117.27 ± 9.32 |
| P.aeruginosa | 8.35 ± 1.01 | 9.35 ± 0.37 | 136.77 ± 10.75 |
|
Corynebacterium spp. |
10.08 ± 0.33 | 9.83 ± 0.83 | 130.46 ± 8.84 |
|
Proteus spp. |
9.82 ± 0.78 | 8.57 ± 0.71 | 110.5 ± 7.69 |
| Normal | 6.35 ± 0.29 | 11.66 ± 1.13 | 75.4 ± 7.38 |
P. multocida were susceptible to ceftiofur, fluoroquinolones and differed in their susceptibility to ampicillin, amoxicillin+ clavulanic acid, and tetracycline. Moreover, the current results somewhat agreed with Härtel et al. (2004) who found that Pasteurella spp. showed no resistance to enrofloxacin or ciprofloxacin but differed in the susceptibility to other antimicrobials including ampicillin, penicillin, and tetracyclines. On the contrary, these results disagreed with those reported by Abera et al. (2014) in Western Ethiopia, who detected the antimicrobial susceptibility of P. multocida and M. haemolytica isolates and found that the isolates were susceptible to most of the antibiotic discs used including amoxicillin, chloramphenicol, cephalexin, kanamycin and florfenicol. However, moderate resistance was observed to tetracycline, erythromycin and penicillin-G. The obtained resistances against tetracyclines, penicillins and aminoglycosides might be attributed to the miss use of antimicrobial in animal treatment.
Nitric oxide releasing solution (NORS) has potential in the prevention of BRD (Edwards, 2010). It protects against the development of BRD by limiting harmful inflammatory effects while simultaneously increasing and enhancing the ability of the host to detect and respond to bacterial pathogens (Sheridan et al., 2016). NO radicals are produced by leukocytes (Rodenas et al., 2000) and acts as intracellular signaling molecule or as neurotransmitter when produced in low quantities and when is produced in high quantities for extended periods, it kills microorganisms and tumor cells.NO was identified as the effector molecule in killing a wide range intra and extracellular pathogens (Schmidt and Walter, 1994).
Lysozymes are naturally found in body secretions such as tears, saliva as well as milk and chicken egg white. It is a part of the nonspecific opsonin response associated with defense against bacteria (Oien and Moskovitz, 2007). Their antimicrobial function is due to cleavage of the peptidoglycan component of bacterial cell walls leading to cell death (Oliver and Wells, 2015). Surprisingly, lysozymes show antibacterial effects even after irreversible inactivation (Ramos and Malcata, 2011).
Interleukins are very important in the key functions of the immune system, tolerance and immunity, primarily via its direct effects on T cells (Liao et al., 2011). The activated macrophages produce important cytokines as IL-6which are contributing to host defense by inducing fever and termed endogenous pyrogens. Expression of cytokines has a consistent up regulation in pneumonic cattle. They can participate in the immune and inflammatory responses during the pulmonary defense mechanisms (Rodríguez et al., 2015). Acute inflammatory cytokines such as IL-6 activate endothelial cells, epithelial cells, alveolar macrophages and lung dendritic cells leading to release of chemokines attracting neutrophils and monocytes into the affected area and with time also attract T and B cells (Ackermann et al., 2010).
On the immunologic level, data of the existing study illustrated in Tables 8, 9 and 10 showed that all respiratory affected calves recorded significant elevations of both serum NO and IL-6 levels compared with normal control calves while elucidated a significant reduction of lysozyme activity.
Regarding NO, this result agreed with those recorded by Civelek et al. (2007) who recoded that serum NO significantly increased in pneumonic calves. Serum NO is a well-known key mediator in sepsis. Also, Tracey et al. (1995) and Lorenteet al. (1997) found that plasma nitrite and nitrate concentrations significantly increased in an animal model characterized with endotoxaemia. Moreover, Hermeyer et al. (2012) published a study of lung sections of the examined calves. They indicated that the production of NO and peroxynitrite is potentially involved in the development of necrotizing lung lesions. Increasing concentrations of peroxynitrite is leading to the generation of reactive oxygen and nitrogen spp. (ROS and RNS) which both have cytotoxic capacities. Therefore, both ROS and RNS are potentially involved in the development of severe necrotizing lung lesions seen in the animals of this study.
Ackermann et al. (2012) found that serum lysozyme level suppressed in pneumonic animals. Moreover, several studies have examined lysozyme’s potential as an exogenously administered bio-therapeutic. Bhavsar et al. (2011) administered aerosolized recombinant lysozyme as a treatment for P. aeruginosa lung infection in hamsters. They found that 2 hrs of treatment for 3 consecutive days decreased the bacterial burden in both broncho-alveolar lavage fluid (BALF) and lung homogenate. The enzyme treatment also decreased lung tissue inflammation, reduced BALF leukocytes and neutrophils, and decreased alveolar septal apoptosis (Teneback et al., 2013). These findings might be attributed to that Neutrophil–derived lysozymes play a major role in intracellular destruction of ingested bacteria, through the formation of phagolysosomes with primary and secondary granules (Seki et al., 2004).
Throughout the current investigation IL-6 elucidated a significant augmentation in infected calves with different levels according to the causative isolate. These results run parallel to the study of Kabu et al. (2016) who revealed that the serum concentrations of cytokines (IL-1β, IL-6 and TNF-α) were high in respiratory affected calves compared with the control group. Similar results were also obtained by Alam et al. (2018) who recorded a significant increase a high IL-6 level in pneumonic male New Zealand rabbits infected with P. multocida. The recoded data might be referred to P. multocida toxin (PMT) which is the major virulence factor and stimulated 3T3 cells to release IL-6 (Rosendal et al., 1995). The role of IL-6 in inflammation depends on induction of the acute phase response, triggering T-cell proliferation and stimulating the differentiation of B-cells (Yoshida et al., 2010; Rosser et al., 2014). In this dissertation, IL-6 will be proven as a prognostic marker in neonatal calf diarrhea. As very little information concerning IL-6 in newborn calves exists, Yoshida et al. (2010) investigated the physiological development of IL-6 gene-expression and serum titers during the first four weeks of life. Rosser et al. (2014) in another study investigated IL-6 in calves suffering from diarrhea to determine the prognostic reliability of this parameter in predicting the course of the disease.
Conclusion
It was concluded that bovine respiratory disease is a serious problem affecting beef calves. P. multocida and M. haemolytica were the most commonly isolated bacteria in such affections. The in-vitro sensitivity testing of such isolates showed high susceptibility to fluoroquinolones and cephalosporins while high resistances were observed against tetracyclines, penicillins and aminoglycosides. On the immunologic level, the data of the existing research work showed that all respiratory affected calves recorded significant elevations of serum nitric oxide andIL-6levels compared with normal control calves while elucidated significant reduction of lysozyme activity.
Acknowledgments
The authors would like to thank the Bacteriology, Mycology and Immunology Department staff at the Faculty of Veterinary Medicine, Beni-Suef University and staff in Animal Reproduction Research Institute, Giza, Egypt, for providing technical help.
Authors Contribution
All authors contributed equally in the planning of the study, drafting the manuscript. All of them approved the final version of the article.
Conflict Of Interest
The authors declared there is no conflict of interest.
References