Advances in Animal and Veterinary Sciences
Research Article
Virological Investigation of Bovine Herpes Virus 1 and Bovine Herpes Virus 4 Infections in Cattle with Endometritis in Kars Province of Turkey
Volkan Yılmaz*1, Nüvİt Coşkun1, Mushap Kuru2, Semra Kaya2, Fatİh Büyük3, Özgür Çelebi3
1Department of Virology, Faculty of Veterinary Medicine, Kafkas University, Kars, Turkey; 2Department of Obstetrics and Gynecology, Faculty of Veterinary Medicine, Kafkas University, Kars, Turkey; 3Department of Microbiyology,, Faculty of Veterinary Medicine, Kafkas University, Kars, Turkey.
Abstract | Bovine herpes virus type 1/4 (BoHV-1/4) shows affinity especially to the respiratory and genital system and can cause economic losses by adversely affecting animal health. The purpose of this study was to identify the presence of BoHV-1 and BoHV-4 in leukocyte samples using molecular methods. Therefore, leukocyte samples were collected from 100 cattle with endometritis, not immunized against these infections, in small-scale family farms in the province of Kars, Turkey. The presence of BoHV-1 and BoHV-4 nucleic acids was investigated with polymerase chain reaction (PCR) specific for the Glycoprotein C (gC) and Glycoprotein B (gB) gene, respectively. BoHV-1 nucleic acid could not be detected in any of the leukocyte samples. However, BoHV-4 nucleic acid was detected in 42 cattle with endometritis. Data obtained from this study and identification of the BoHV-4 specific antigen in leukocyte samples in the province of Kars proves that the BoHV-4 infection is present in the region and plays an important role in cases of endometritis observed in cattle.
Keywords | BoHV-1, BoHV-4, Endometritis, Kars, PCR
Received | October 30, 2019; Accepted | April 13, 2020; Published | May 02, 2020
*Correspondence | Volkan Yilmaz, Department of Virology, Faculty of Veterinary Medicine, Kafkas University, Kars, Turkey; Email: volkankara1980@hotmail.com
Citation | Yilmaz V, Coskun N, Kuru M, Kaya S, Buyuk F, Celebi O (2020). Virological investigation of bovine herpes virus 1 and bovine herpes virus 4 infections in cattle with endometritis in kars province of turkey. Adv. Anim. Vet. Sci. 8(5): 531-535.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.5.531.535
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Yilmaz et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Bovine herpesvirus 1 (BoHV-1) is classified in the Varicellovirus genus in the subfamily Alphaherpesvirinae of the family Herpesviridae. There are three subtypes of BoHV-1. Subtypes 1 and 2a cause respiratory symptoms and abortions. Subtype 2b results in Infectious Pustular Vulvovaginitis/Infectious Balanopostitis (IPV-IPB) characterized by genital lesions (Yilmaz et al., 2016). The clinical onset of the infection changes with the subtypes of virus, virulence and the immune status of the host (Queiroz-Castro et al., 2019). The BoHV-1 virion is 150-200 nm in diameter has an icosahedral capsid consisting of 162 capsomeres, and it is enveloped. It has an infectious genome with a linear double strand of 136 kbp, containing at least 65 genes. The entry of the virus into the cell is governed by membrane glycoproteins (gB, gC, gD). Like other herpes viruses, BoHV-1 becomes latent in the trigeminal and sacral ganglia following primary infection, and reactivates later in stressful conditions like pregnancy, transplantation, corticosteroid administration etc. The recurrent infection period includes infective virus scattering with clinical findings (Kaddour et al., 2019).
BoHV-4 is an agent classified in the Gammaherpesvirinae subfamily of the Herpesviridae family and in the Rhadinovirus genus. The agent has an icosahedral nucleocapsid having a diameter of 150 nm and contains double stranded DNA. The antigenic and biological relation of BoHV-4 with other common bovine herpesviruses has not been determined (Roizman et al., 1992). Clinically, it can cause mastitis, peritonitis, vulvovaginitis, metritis, dermatitis, respiratory problems and abortions (Wellenberg et al., 2001), and it also can be isolated from materials of healthy-looking cattle (Yang et al., 2019). Endometritis causes economic losses due to decreasing reproductive performance, increasing feed intake, decreasing milk yield. It also affects the animal welfare negatively.
The PCR technique used to detect BoHV-4 viral DNA is a specific and sensitive method. Glycoprotein B is an essential protein in viral infectivity and is the most important component of virion for BoHV-4 (Lomonte et al., 1997). This indicates that the gB gene is present in all BoHV-4 strains. Wellenberg et al. (2001) found that viral DNA can be detected by gB PCR when there is 2-10 BoHV-4 DNA copies in a clinical sample. The same researchers reported the sensitivity of the gB PCR and virus isolation methods as 93% and 61%, respectively.
Endometritis is important problem in cattle in both the North-eastern Anatolia and Turkey. Especially, endometritis rate caused by viral agents is dramatically increasing in the recent years. This study investigated the presence of BoHV-1 and BoHV-4 nucleic acids in leukocyte samples obtained from small-scale family farms in the province of Kars, Turkey. PCR was used for the detection of gC and gB genes of BoHV-1 and BoHV-4, respectively. This study was performed to assess the etiological role of the virus in the cases of endometritis
MATERIAL AND METHODS
Ethics Statement
This research was conducted after the approval of Kafkas University Animal Experiments Local Ethics Council (Approval Number: KAU- HADYEK-2015-070).
Study Area and Sample Collection
This study was conducted in Kars region in Northeast part of Turkey. The Kars region, located in Northeastern Turkey (43.05° E and 40. 36° N), which is the most important livestock production area in Turkey. It is a mountainous area and has a cold climate (Figure 1). Leukocyte samples were obtained from 100 cattle with clinically endometritis aged above 3 years between November in 2017 to March in 2018 from small scale family farms (less than twenty cattle) in Kars district in Turkey. The cattle included in the study were not vaccinated against BoHV-1 and BoHV-4. The diagnosis of endometritis was done by rectal and vaginoscopic examination. In these examinations, the appearance of the uterus, the character and quantity of uterine influx were considered. Blood samples were taken from the vena jugularis to EDTA tubes. The peripheral blood samples in EDTA tubes were centrifuged at 2000 x rpm for 20 min and leukocyte fractions were collected with Pasteur pipettes and resuspended in 2 ml PBS.
DNA extraction and PCR Technique
From leukocyte samples, viral DNA was extracted using a GeneJet Viral DNA and RNA Purification Kit (Thermo Scientific, Waltham, MA, USA), according to the manufacturer’s recommendation. The detection of BoHV-1 DNA was performed using the BoHV1-glycoprotein C (gC) PCR as described by Van Engelenburg et al. (1993). The primer pair (P1: 5’-CTGCTGTTCGTAGCCCACAACG-3’), and (P2: 5’-TGTGACTTGGTGCCCATGTCGC-3’), produced a 173 bp fragment. The detection of BoHV-4 DNA was performed using the BoHV4-glycoprotein B (gB) PCR as described by Wellenberg et al. (2001) with some modifications. The primer pair (B1:5’- CCCTTCTTTACCACCACCTACA-3’) and (B2:5’- TGCCATAGCAGAGAAAC AATGA-3’) produced a 615 bp fragment. Briefly, 3 µl DNA was subjected to thermocycling in a 30 µl reaction mixture. The reaction mix contained 2.5 U Taq Polymerase, 3.5 mM dNTP mix, primers at 10 pmol concentrations, 1.5 mM MgCl2, 1X PCR buffer, 6% DMSO and DNase/RNase free water. Thermal cycling conditions were 6 min at 96°C followed by 40 cycles at 95°C for 1 min, 56°C for 45 sec, 72°C for 2 min and followed by a final 10 min extension at 72°C for both primer sets. Five microlitres of each PCR product were analyzed on 1% agarose (Prona, Spain) gel containing ethidium bromide (Sigma, USA). The gels were read for specific size bands by UV transillumination. BoHV-4 DN-599 strain and BoHV-1 Cooper strain were used as control viruses for PCR. Reference virus strains were kindly obtained from the Ankara University Faculty of Veterinary Medicine Virology Department.
RESULTS AND DISCUSSION
A total of 100 leukocyte samples were analyzed by PCR. BoHV- 4 DNA was detected in 42% (42/100) of leukocyte samples. The expected amplicon size of 615 bp product of BoHV-4 was observed in positive samples (Figure 2). None of 100 cattle leukocyte samples were positive for BoHV-1 DNA.
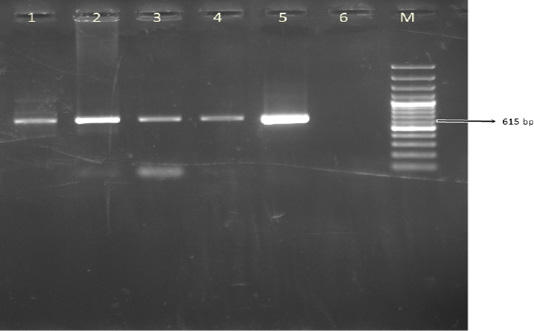
Figure 2: The result of BoHV-4 gB PCR in leukocyte samples. Line M. 100 bp DNA ladder, Lines 1-4. Positive samples, Line 5. DN-599 (BoHV-4 reference strain, 615 bp), Line 6. Negative control
There are many bacterial and viral agents that can cause fertility problems and abortions with causing clinical findings of the genital system (Aslan et al., 2015; Timurkan and Aydin, 2019). Repeat breeding due to metritis, oophoritis, early embryonic death and abortion due to generalized infections are the causes of the major economic losses. Therefore, the determination of the etiological agent in the genital tract problems and abortions is important in revealing the control and protection measures against these causes. Belonging to herpesviridae family, BoHV-1 and BoHV-4 is two of the viral agents that are common in cattle and cause economic losses due to different clinical manifestations. Herpesvirus infections in cattle must be investigated using laboratory methods following suspicious clinical diagnosis. The diagnosis of these infections is important for the implementation or development of control programs related to the identified infection.
BoHV-1 infection is still outspread worldwide (Ferreira et al., 2018; Mendes et al., 2018), but eradication studies have been performed and being performed in many European countries. BoHV-1, is reported to have high seroprevalance in cattle in Turkey, ratios of up to 100% have been reported (Alkan et al., 1997; Yavru et al., 2001; Tan et al., 2006; Yildirim et al ., 2009). Cabalar (1993) investigated the role of BoHV-1 infection in Turkey’s different regions where fertility problems exist in cows and found seropositivity rate of 68.1% despite not being able to isolate the virus. Özkul et al. (1995) reported that BVDV and BoHV-1 caused a combination infection in 45.9% of the cows with genital tract problems investigated in 19 dairy cattle farms.
In a few European countries such as Denmark, Sweden and Finland, which take place in the European Union, vaccination is prohibited, and a program based on the removal of seropositive animals for BoHV-1 specific antibodies from the herds is carried out to eradicate the infection. In some countries such as Austria and the Netherlands, a national eradication program based on vaccinating with a marker vaccine. This allows distinguishing of naturally infected animals and animals vaccinated with marker vaccines. Naturally infected animals are removed from herds at an economically acceptable level (Kofer et al., 1999; Van Schaik et al., 2001).
BoHV-4 infection was detected in Africa, several European countries and the United States (Mohanty et al., 1971; Truman et al., 1986; Van Malderen et al., 1987; Graham et al., 2005; Monge et al., 2006). The presence of BoHV-4 infection in different clinical symptoms has been reported (Fitton et al., 1990; Wellenberg et al., 2000; Graham et al., 2005; Monge et al., 2006) but could not be associated with the role as etiological agent. In a comparative study in animals with and without genital duct problems, Biuk-Rudan et al. (1998) found that the seroprevalence of BoHV-1 was 80.8% in animals with genital canal problems and in 46.8% of patients without genital canal problems. Frazier et al. (2002) reported endometritis cases associated with BoHV-4 in the USA. Monge et al. (2006) found that BoHV-4 was an important factor in postpartum metritis cases in Spain. Deim et al. (2007) reported that the virus plays an active role in genital tract problems. Wellemans et al. (1986) reported that after experimental inoculation of BoHV-4, long-lasting metritis was observed in cattle and deaths with unknown etiology were detected in the study. Wellenberg et al. (2001) found that BoHV-4 was an important factor in mastitis cases. Izumi et al. (2006) reported BoHV-4 detection in teat lesions.
Bilge Dagalp et al. (2010) demonstrated the presence of BoHV-4 infection serologically and virologically in a farm with metritis problems in Turkey. Bilge Dağalp et al. (2012) investigated the presence of BoHV-1 and BoHV-4 nucleic acid in 13 different dairy cattle farms using gC PCR and gB PCR techniques in leukocyte samples, vaginal swabs and waste fetuses belonging to cattle with reproductive disorders. While BoHV-1 was not detectable, positivity for BoHV-4 gB gene was 26.1% and 33.6% in vaginal swab and leukocyte samples, respectively. Sevik and Avci (2015) tested organ samples (lungs, liver and spleen, total n = 198) of aborted small ruminant fetuses (43 sheep and 23 goats) with glycoprotein D (gD) gene-specific PCR technique and were not able to detect BoHV-1 presence.
In this study, PCR was used for the detection of gC and gB genes of BoHV-1 and BoHV-4, respectively.. As a result of our study, BoHV-1 could not be detected in cattle with endometritis. In contrast, 42% (42/100) of the samples tested for BoHV-4 nucleic acid presence were found to be positive. It is thought that the lack of detection of BoHV-1 nucleic acid in leukocyte samples may be related to negativity in transport conditions, fragility of virus, low viral load in leukocyte samples and short term viremia of the samples . BoHV-1 can remain latent by entering the trigeminal and sacral ganglions after the primary infection (Roizman et al., 1992). In this study, it was thought that Ag positivity could not be detected by PCR due to the latency of BoHV-1.
BoHV-4 has been shown to cause persistent infection and suppress the immune response (Klamminger et al., 2017). This effect of the virus affects the defense of animals against other pathogens during acute and latent BoHV-4 infection. In some cases, BoHV-4 causes persistent infection in leukocytes, splenic macrophages and endothelial cells (Tebaldi et al., 2016; Klamminger et al., 2017; Yang et al., 2017) and may be reactivated in tissues by administration of dexamethasone (Castrucci et al., 1987). Researchers (Wellemans et al., 1986; Monge et al., 2006) also reported that pregnancy may cause BoHV-4 reactivation and emphasized the importance of this in the onset of postpartum metritis.
Consequently, this study demonstrates that BoHV-4 plays an important etiological role in cases of endometritis. Small family farms with endometritis should be periodically checked for viral agents that cause endometritis. Furthermore, genomic identification of the BoHV-4 strains obtained from this study will play an important role in future studies on protection and control strategies to combat and prevent the spread of infection.
ACKNOWLEDGEMENT
This project was supported by the Commission for the Scientific Research Projects of Kafkas University (KAU BAP-2015-TS-81).
conflict of interest
There is no conflict of interest.
Authors contribution
All authors contributed equally.
REFERENCES