Advances in Animal and Veterinary Sciences
Research Article
Effect of Dried and Processed Yoghurt Addition on the Sites of Protein Digestibility along Gastro-Intestinal Tracts of Broiler Chickens
Rabia J. Abbas1, Abdullah A. Mohammed1, Mayssam A. Hussan2
1Department of Animal Production, College of Agriculture, University of Basra, Basra, Iraq; 2Veterinary College, University of Basra, Basra, Iraq.
Abstract | The digestion of dietary proteins provides essential amino acids which are involved not only in the metabolism of body proteins, but also in other metabolic pathways in different organs. The present study was undertaken to evaluate the effect of natural feed additives comprising probiotic (yoghurt powder), phytobiotic (Cinnamon) and B-vitamins loaded with different feed materials, e.g., lentil, yellow corn, and wheat bran on protein digestibility in various sections of the gastrointestinal tract and total acidity of intestinal fluid. A total of 240 day-old broiler (Ross- 308) hatching chicks were randomly distributed on eight treatments with three replicates per treatment (10 chicks per replicate) according to the Complete Random Design., viz., (T1): basal diet (BD) as a control, (T2): BD mixed with yoghurt powder (3g/kg YP) containing cinnamon (Cinnamomum verum) and lentil (carrier), (T3): BD mixed with YP containing vitamin B-complex and yellow corn (carrier), (T4): BD mixed with YP containing cinnamon and yellow corn (carrier), (T5): BD mixed with YP containing vitamin B-complex and wheat bran (carrier), BD supplemented with 0.5g/kg feed of imported probiotic Labzyme, Biolac and Interzyme in treatments T6, T7 and T8 respectively, in terms of protein digestibility of broilers at 35 days of age. The results showed a significant improvement (p˂0.05) in protein digestibility of the dried yoghurt probiotic treatments (T2-T5) in the crops, gizzard, jejunum, ileum and in feces as compared with control and imported probiotic treatments (T6- T8). A significant improvement in lactic acid percentages of dried yoghurt probiotic (T2 to T5) and T6 (Labzyme) as compared with control and imported probiotic (Biolac and Interzyme in treatments T7 and T8) respectively. It is concluded that the diet complemented with yoghurt powder probiotic with cinnamon and B-vitamins was the best natural feed additive for protein digestibility enhancement of different sites of gastrointestinal tract and better lactic acid percent’s of intestinal fluid of broiler chicks.
Keywords | Protein digestibility, Broiler chickens, Dried yoghurt, Carrier materials, Total acidity.
Received | October 30, 2019; Accepted | January 17, 2020; Published | April 15, 2020
*Correspondence | Rabia Abbas, Department of Animal Production, College of Agriculture, University of Basra, Basra, Iraq; Email: rj.abbas@yahoo.com
Citation | Abbas RJ, Mohammed AA, Hussan MA (2020). Effect of dried and processed yoghurt addition on the sites of protein digestibility along gastro-intestinal tracts of broiler chickens. Adv. Anim. Vet. Sci. 8(5): 484-489.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.5.484.489
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Abbas et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Proteins are essential substances in the synthesis of all living cells and are produced by intensification of a long number of different amino acids. These amino acids are the main part of the living material in the cells, therefore, protein compounds must be broken down into their constituents amino acids to facilitate their passage through the small intestine walls (Ishibashi and Yonemochi, 2002). Digestibility is probably the most important factor of the efficiency of feed utilization, and it is inherent features of feeds to a large extent (Ravindran and Bryden, 2003). Noreen (2006) reported that the determination of feed nutrient digestibility was also considered as an important parameter in evaluating the nutrient adequacy or availability and efficiency of the feedstuffs. Protein digestibility can be measured in various compartments of the digestive tract (GIT). It represents the quantity of nutrients (amino acids or peptides) disappearing during the digestive processes relative to its entry rate in the studied GIT compartment (Savary-Auzeloux et al., 2014). The digestion of feed with poultry has been generally measured over the total tract digestibility, such evaluation, however do not provide information on the sites of intestinal digestibility. Identification of the sites of the nutrients digestion are critical to understand the dynamic of these nutrients digestions and absorption in poultry is not only limited, but also contradictory. In this regard, Ravindran et al. (1999); Kadim et al. (2002), reported that ileal digestibility is considered to be a more accurate measure of amino acid availability in chickens than the total tract digestibility. Another study stated that the use of artificially prepared enzymes in broiler diets resulted in significant improvement in protein digestion factors compared to enzymes addition treatment (Sherif, 2009). Several studies have shown that the addition of probiotics to broiler chicks feeds leads to improved protein digestibility (Afsharmanesh et al., 2010; Gaware et al., 2011; Agboola et al., 2014). Noy and Sklan (1995) observed that in ileum protein digestion increased from 78 % at 4 days to 92 % at 20 days. Similarly, the probiotics manufactured from 3g milk kefir/kg diets of broiler chickens revealed a significant improvement in protein digestion factor (Auda, 2017). The aim of this study was to determine the digestibility of protein in different section of the gastrointestinal tract inclusion chromic oxide in the diets by using dried yoghurt loaded with lentils with cinnamon, yellow corn with B-complex vitamin, yellow corn with cinnamon and wheat bran with vitamin B-complex as probiotics and compare it with the imported probiotic.
MATERIALS AND METHODS
Animal Husbandry and Treatments
This experiment was conducted at the Poultry Research Unit of the Animal Production Department, College of Agriculture, University of Basra (Iraq) between 2 January and 8 February 2017. A total of 240 unsexed one day of the Ross-308 broiler chickens at rates of 40 g chicks were distributed randomly among eight treatments, three replicates per treatment of 10 birds each. All replicates were reared in floor cages supplement with feeders and drinking system.
Supplements
Probiotic yoghurt powder (YP) used in the current study were prepared in Microbiology Laboratory, Animal Production Department of the College of Agriculture, and incorporated into broiler basal diets as in the method previously reported by Naji et al. (2012). The probiotic yogurt was synthesized by mixing 990 g of carrier material (lentil flour / ground yellow corn / wheat bran flour) with 8 g yogurt powder and 2 g (cinnamon powder or B-complex vitamins). Then, chicks were fed on basal diet supplemented with probiotic yogurt at 3g/kg feed.
The first treatment (T1) was fed on basal diet without any supplementation (control). Treatments T2, T3, T4 and T5 a basal diet supplemented with 3 g of dried yoghurt loaded on lentils with cinnamon, yellow corn with B-complex vitamins, yellow corn with cinnamon and wheat bran with vitamin B-complex respectively/kg diet. Treatments T6, T7 and T8 a basal diet supplemented with 0.5 g imported probiotic of Labzyme, Biolac and Interzyme respectively/kg diets. Basal diet was formulated and compounded to meet the nutrient requirements of commercial broilers (NRC, 1994) during the starter stage (1-21 days) and grower stage (22-35 days) are shown in Table (1) Chicks had a free access to feed (mash) and water entire the experimental period. The inclusion rates of yoghurt powder (YP), lentil flour, ground yellow corn, wheat bran, cinnamon, and B-vitamins are shown in Table (2).
Table 1: Ingredient and chemical composition of starter and finisher diets
|
Ingredient |
Starter diet 1-21 day |
Finisher diet 22-35 day |
| yellow corn | 42.75 | 41.75 |
| Wheat | 15.00 |
22.00 |
|
Soybean meal (44%) |
34.00 |
27.00 |
| protein concentrate | 5.00 | 5.00 |
|
Vegetable oil |
0.80 |
2.30 |
| Premix | 0.20 | - |
| Limestone | 1.50 |
1.70 |
|
Common salt |
0.25 |
0.25 |
|
Total |
100 | 100 |
| Calculated composition | ||
|
*ME (Kcal /Kg diet) |
3010 | 3174 |
|
Crude protein (%) |
23.10 | 20.14 |
|
Calorie: protein ratio |
130.30 | 157.60 |
|
Calcium (%) |
0.93 | 0.99 |
|
Phosphorus available (%) |
0.42 | 0.51 |
|
Lysine (%) |
1.35 | 1.17 |
|
Methionine + Cystine (%) |
0.89 | 0.83 |
*ME: Metabolizable energy
Protein Digestibility Measurements
Six chicks (3 male and 3 female) were separated from each treatment for the age of 35 days and kept separately for up to 38 days for protein digestibility measurement. They were fed the same grower diets mixed with 2g chromic oxide /kg diet for three days. Three birds were slaughtered and their gastrointestinal tract (GIT) was quickly removed. The length of GIT was determined prior to separate into the following section, crop, gizzard, jejunum, and ileum. On days 37 to 38, total feces voided were collected from each individual bird for protein utilization evaluation.The contents of these sections were collected and transfer to ovens to dryness at 105 0C for 24 hours (AOAC, 2006). Finally these samples sections were ground, then kept in small plastic jars to measure the crude protein percent using the methods as described by AOAC (2006). The same samples were used to determine the chromium by Atomic absorption photometry. The digestibility of nutrients was calculated by means of the following formula described by Khan et al. (2003):
Digestion coefficient of a nutrient % =
Indicator in feed %× Nutrient in feces %
ــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــ x 100 -100
Indicator in feces % × Nutrient in feed %
Total Acidity
Six samples of intestinal fluid from broiler chicks were used to measure the lactic acid percentage according to method described by Marth (1978).
Lactic acid % =
(0.1M) NaOH × vol. of NaOH (in liter× 90.08*)
ـــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــــ × 100
Weight of the sample
Where:
*90.08 g/mol= Is the molecular weight of Lactate
Table 2: The inclusion rates of dietary complements of different groups (g/kg)
| Treatments | Dietary Component |
Yoghurt powder (YP) |
| T1 | Basal Diet (Control) | - |
| T2 | Basal Diet + YP + lentil+ cinnamon | 3 |
| T3 | Basal Diet + YP+ yellow corn+ B-vitamins | 3 |
| T4 | Basal Diet + YP+ yellow corn+ cinnamon | 3 |
|
T5 |
Basal Diet + YP+ wheat bran+ B-vitamins | 3 |
|
T6 |
Basal Diet + 0.5 g Labzyme/kg feed | - |
| T7 |
Basal Diet + 0.5 g Biolac/kg feed |
- |
| T8 |
Basal Diet + 0.5 g Interzyme/kg feed |
- |
Statistical Analyses
All data were subjected to one-way- ANOVA procedure of SPSS (2012). Means were compared by Duncan›s (1955) Multiple Range Test (p≤0.05).
RESULTS AND DISCUSSION
Protein Digestibility
A large percentage of the feed ingredients consumed by a chicken is in a form that necessitates chemical and other reactions before it can be utilized by the bird. Digestion refers to those changes that occur in the alimentary canal to make it possible for the feed to be absorbed through the intestinal wall and enter the bloodstream (Coon, 2002).
Figure (1) and Table (3) show that protein digestibility occurred in the crop, gizzard, jejunum, ileum and the feces of the broiler chickens fed the experimental diets. Crops had little protein digestibility in treatments T2, T3, T4, T5, and T6. The values of digestibility were 3.92, 4.18, 3.94, 5.45 and 4.29 % respectively higher little pit than the control treatmentsT1, T7 and T8 (3.81, 3.54 and 3.38 % respectively. The reason for the improved protein digestion was due to the presence of Lactobacilli bacteria in the powdered yogurt, which settled in the compost of the crop and characterized by their effect in the ferment-
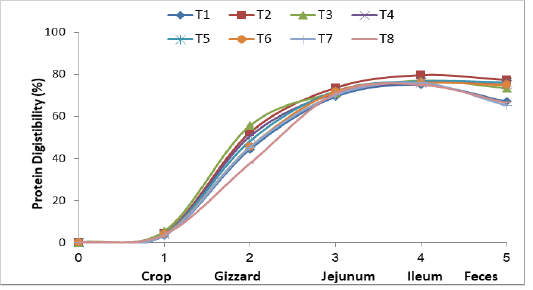
Figure 1: Effect of supplementing dried and processed yoghurt in broiler diets on protein digestibility in gastro-intestinal tract and feces
Table 3: Effect of supplementing dried and processed yoghurt in broiler diets on protein digestibility (%) of the gut contents and feces of broiler at 35 days of age
|
Treatments |
Crop | Gizzard | Jejunum | Ileum | Feces |
| 1T |
3.38 c |
44.40b |
69.43b |
75.08b |
67.08b |
| 2T |
4.29 a |
52.35a |
73.62a |
79.71a |
77.38a |
| 3T |
5.45 a |
55.62a |
71.27a |
77.05a |
73.53a |
| 4T |
3.94 a |
50.59a |
71.03a |
76.18ab |
75.16a |
| 5T |
4.18 a |
48.34a |
72.09a |
76.73a |
76.15a |
| 6T |
3.92 a |
45.50b |
71.86a |
75.94b |
74.98a |
| 7T |
3.54 b |
45.35b |
70.18ab |
75.92b |
65.20b |
| 8T |
3.81 b |
37.68c |
70.89ab |
75.12b |
66.45b |
| SEM | 0.25 | 1.94 | 0.51 | 0.46 |
2.33 |
|
P< |
|
|
|
|
|
Notes: a, b,c Means in the same row with no common superscript are different significantly (p≤0.05), SEM: Standard Error of Means
ation processed, which dissolved a small percentage of amino acids of some proteins during storage (Green and Sainbury, 2001). The protein digestibility was significantly higher in the gizzard, treatments T2, T3, T4 and T5 were protein digestibility 52.35, 55.62, 50.59 and 48.34 % as compared with controls, T6, T7 and T8 (44.40, 45.50, 45.35 and 37.68 %) respectively. This elevation was due to the fact that the upper parts of the digestive system of the domestic birds, including the compass are characterized by a high acidity level, which encourages the colonization of certain bacteria like Lactobacilli and Enterococcus. Hilmi et al. (2007) indicated that Lactobacillus and Enterococcus were dominated in the upper part of intestine treatments in broiler chickens. The birds have proventriculus and gizzard for gastric digestion, the lining of the proventriculus contains papillae and multi-lobular gland, which secrete both acid in form hydrochloric acid and proleolytic enzymes in the form of pepsin (Kleyn, 2013). Pepsinogen under the influence of high acidity, the pepsinogen transform into active pepsin and works on the decomposition of proteins, the gizzard acts only as a mechanical organ, it is important for mixing ingested feed with water, saliva, hydrochloric acid and pepsin, also the time spent in the gizzard by the digesta allows for increased contact between the feed and gastric acid and pepsin therefore facilitating the further denaturation and digestion of proteins prior to release into the small intestine (Rynsburger, 2009). The same figure shows the high protein digestion in the jejunum of treatments T2, T3, T4, T5 and T6 at 73.62, 71.27, 71.03, 72.09 and 71.86 % respectively as compared to T1, T7 and T8, its reported 69.80, 70.18 and 70.89 respectively. This is due to enhancement of the efficiency of enzymes in the duodenum and small intestine, such as peptidase and proteinase from pancreas which stimulated by cholecystokinin (Amerah et al., 2009), for the sovereignty of beneficial bacteria (Flint and Garner, 2009). The higher digestion rate in the ileum of protein in treatments T2, T3, T4 and T5 with dried yoghurt supplemented. It was recorded 79.71, 77.05, 76.18 and 76.73 % compared with the control (75.08 %) and the treatments added to the imported probiotics T6, T7 and T8, which reached 75.94, 75.92 and 75.12 % respectively. The reason for improvement of the digestion of the protein to the acidity level in the gastrointestinal tract was due to it’s importance in influencing protease enzyme which showed the rise of lactic acid in the contents of small intestine. The decrease in pH showed at Table (4), stimulates and facilitates the enzyme’s activity as well as it’s active involvement in the decomposition of feed which prevents the growth of pathogenic and proved a greater changes for the sovereignty of beneficial bacteria (Flint and Garner, 2009; Recoules et al., 2017). Noy and Sklan (1995), also showed an improvement in the nitrogen digestion rate of 92 % in the ileum area. The overall digestibility in the feces showed that highly apparent digestion of the birds treatments T2, T3, T4, T5 and T6 fed on the yoghurt supplements and Labozyme, which was 77.38, 73.53, 75.16, 76.15 and 74.98 % respectively compared with control (67.08 %) and treatments T7 and T8 which supplemented with imported Biolac (65.20 %) and Interzyme (66.45%). The increase in the digestion of protein in general was due to the diversity of microorganisms in the probiotic of dried yoghurt (AL-KhalidiI, 2005). Omara, (2012) indicated that increased in the crude protein digestion when using screening milk and why as natural probiotics. The results of the study agreed with the Auda, (2017) when used of dried kefir milk loaded on feed materials, which resulted in a significant improvement in the digestion of protein in the bird dropping.
Table 4: Effect of supplementing dried and processed yoghurt in broiler diets on lactic acid ratio in the intestine liquid of broiler at 35 days of age
|
Treatments |
Lactic acid (%) | The increase and decrease percent |
| 1T |
45.00b |
0.00 |
| 2T |
60.20a |
33.73 |
| 3T |
65.40a |
45.33 |
| 4T |
62.60a |
39.11 |
| 5T |
67.70a |
50.44 |
| 6T |
60.12a |
33.60 |
| 7T |
50.14b |
11.42 |
| 8T |
48.43b |
7.62 |
|
SEM |
.651 |
- |
|
P< |
0.05 | - |
Notes: a, b,c Means in the same row with no common superscript are different significantly (p≤0.05), SEM: Standard Error of Means
Total Acidity
Data in Table 4 show that there was significant (p≤0.05) improvement in lactic acid percent in treatments T5, T3, T4, T2, and T6 which reached 67.7, 65.4, 62.6, 60.2, 60.12 % respectively, as compared to treatments T1, T8 and T7 that recorded (45.0, 48.43 and 50.14 % ) respectively. The increase in lactic acid percentages of treatments supplemented with YP, could be due to that digestive tracts of chicks contains many types of beneficial bacteria, the most important ones are the lactic acid bacteria (Lactobacilli) , which has a high production of lactic acid (Vesa et al., 2000). Besides, the role of bioactive compounds in yogurt such as organic acids which helps in the secretion of digestive enzymes that work on the hydrolysis of proteins and conversion to simpler materials such as peptides and free amino acids and as a result increase the proportion of lactic acid in the gastrointestinal tract of birds (Tunick and Van Hekken, 2014).
CONCLUSION
The finding of current study contributed to total acidity, a significant elevation of lactic acid present was being recorded at yoghurt probiotic supplemented diets. The jejunum and ileum was the major intestinal sites of protein digestibility.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the Department of Animal Production College of Agriculture University of Basra for logistic support.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
REFERENCES





