Advances in Animal and Veterinary Sciences
Research Article
Seroprevalence of Trypanosoma evansi in Dromedary Camels from selected Dairy farms in Benadir, Somalia
Mohamed Abdelrahman Mohamed1*, Abdullahi Abdi Mohamoud1, Hamdi Ibrahim Adow1, Asinamai Athliamai Bitrus2
1Faculty of Veterinary Medicine asnd Animal Husbandry, Somali National University, Mogadishu, Somalia; 2Department of Veterinary Microbiology and Pathology, Faculty of Veterinary Medicine, University of Jos, PMB 2084 Jos, Plateau Nigeria.
Abstract | This study was conducted to determine the seroprevalence of Trypanosoma evansi and their risk factors in some selected Camel Dairy farms in Benadir, Somalia. The study was conducted from July, 2018 to February, 2019. Simple random sampling was used and the studied animals were selected based on the population of Camel in each farm in the study area. A total of 200 blood samples were collected from six farms that comprised of Goorsan (36), Cagarey (31), Alkhalil (25), Albaraka (60), Mandeq (26) and Sahan (22). Blood serum was harvested and detection of antibodies against T. evansi was carried out using Card Agglutination Test for Trypanosomiasis (CATT). Of the 200 serum samples analyzed, 129 (64%) samples were positive for T. evansi. Farm level seroprevalence showed that Cagarey farm, had the highest seroprevalence [25 (80%)] followed by Goorsan 27(75%), Mandeq 16 (61%), Albaraka 36(60%), Sahan 12(55%) and Alkhalil 13 (52%) respectively. Risk factor analysis showed that there was a statistical significant difference between age, sex, body condition, farms type and the seroprevalence of T. evansi (P<0.05). Higher seroprevalence of T. evansi was recorded in camels >3 years of age (67%) compared to camels between 1-2 years old (54%). Seroprevalence of T. evansi was relatively high in females (66%) than males (44%). Camels with good and or normal body condition had a seroprevalence of 121 (65%) than those with poor body conditions 8 (50%). The results of this study affirmed the occurrence of T. evansi among camels in Benadir region of Somali.
Keywords | Camel, Seroprevalence, Somalia, Trypanosoma evansi
Received | October 30, 2019; Accepted | January 17, 2020; Published | March 05, 2020
*Correspondence | Dr. Mohamed Abdelrahman Mohamed, Faculty of Veterinary Medicine and Animal Husbandry, Somali National University, Mogadishu, Somalia; Email: aamiin33@hotmail.com
Citation | Mohamed MA, Mohamoud AA, Adow HI, Bitrus AA (2020). Seroprevalence of Trypanosoma evansi in dromedary camels from selected dairy farms in Benadir, Somalia. Adv. Anim. Vet. Sci. 8(3): 333-338.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.3.333.338
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Mohamed et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Livestock contributes about 40 % of the global value of agricultural output and in addition to supporting the livelihoods and food security of an estimated 1.3 billion people. The livestock sector is one of the fastest growing arms of the agricultural economy. Livestock is the world’s largest user of land resources, with grazing land and cropland dedicated to the production of feed representing almost 80 % of all agricultural land (Thornton, 2010).
The world camel population is believed to be conservatively around 25.89 million, out of which 89% are one- humped dromedary camels (Camelus dromedarius) and the remaining 11% are the two-humped, Camelus bactrianus (Abera et al., 2015). The importance of camels (Camelus dromedarius) is coming from the fact that it is sustainable agricultural resources for millions of people in the arid and semi-arid zones. Additionally, the exportation of camels contributes to foreign currency earnings of many African countries (Adam, 2017). Somalia has an estimated six million one-humped camel and about 1. 2 million are found in the southern zone of the country (FAO, 2004, 2012). The dromedary is an important livestock species and plays a vital role in national economy and food (Abdurahman et al., 1991; FAO, 2013). They also have social and cultural importance to the pastoralists and for payment of bride-wealth (yarad) and compensation of injured parties in tribal feuds (mag) (Elmi, 1989). To the Somali pastoralist, camel is the most valuable animal and having large herd is a sign of prestige and strength. Camels are not primarily disposable income as they have a great potential for survival in long periods of drought as a recurrent phenomenon in the country (Tale et al., 1993).
Trypanosomiasis is one of the major threats to livestock production in emerging economies of the world. The disease is the single most important cause of economic losses in camel production causing morbidity up to 30% and mortality of around 3% (Nneka CEF and Kojo BSA 2005). Economic losses arise due to reduction in quality of milk, meat, hide and skin, abortion and death (Maina et al., 1995; Njiru et al., 2002; Elhaig and Sallam, 2018). Trypanosomiasis caused by T. evansi is endemic in most tropical zones around the globe, especially in Africa, Latin America and Asia. T. evansi mostly affects camels and horses and the pathogen is transmitted mechanically by biting flies. It has also been reported in other animal species such as goats, sheep, cattle, donkeys and buffalos (Urquhart et al., 1996; Uilenberg, 1998; Tesfaye, 2002; Elhaig and Sallam, 2018).
Trypanosoma evansi is mechanically transmitted via the bites of blood sucking flies such as Stomoxys and Tabanus. This means of transmission is due to the absences of a deficient gene necessary for the development of mitochondria. In some instances, blood sucking insects like Glossina can also serves as a mechanical vector for T. evansi in areas where they co-exist. Key factor to the success of mechanical transmission of T. evansi, is interrupted feed. Flies moves within a short burst from one host to another to transmit the trypanosomes before the parasite die. This is because trypanosomes do not survive for more than 15 minutes inside the proboscis of the fly (Kassa et al., 2011; Desquesnes et al., 2013; Eshetu et al., 2013).
In Somalia, despite the presence of large camel population and its economic and social importance to the pastoral and agro-pastoral communities (FAO, 2004). Livestock management and control programs for infectious diseases like Trypanosomiasis have declined due to lack of central veterinary services in the country. There is also a paucity of data on the prevalence of T. evansi in Camel. Therefore, the aim of this study was to determine the seroprevalence of T. evansi and their associated risk factors among camels from selected dairy farms in Benadir, Somalia.
MATERIALS AND METHODS
Study design
This is a cross-sectional study designed to estimate the seroprevalence of T. evansi in camel and evaluate the risk factors associated with their occurrence.
Study population
A total of 200 indigenous breeds of camels (one-hump camel) of different ages and sex, reared under semi-intensive management system were sampled. The age of camels was determined based on dentition and farm records, the animals were grouped into two; young (1-2) years old and adult (> 3) years old.
Study area
The study was carried out in Benadir, the region lies between latitude 2.046934 and longitude 45.318161. The average annual temperature ranges between 28.7˚C - 37˚C. The region has the largest population across Somalia and it is estimated to have about 2.3 million people and covers an area of approximately 96,878 km (Wikipedia, 2018).
Sampling method and sample size determination
The studied camels were selected using simple random sampling method by considering the age, sex and body condition of the animals. A representative sample size was determined using the formula of Thrusfield (2005).
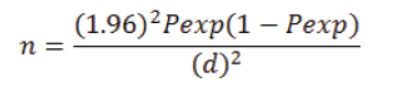
Where; n= Sample size; Pexp= Prevalence expected; d = required precision; which is popularly known to be =0.05 or 5%.
By using expected prevalence of 15% (Mohamoud, 2017).
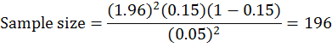
The sample size used in the study was 200 Camels.
Sample collection and examination procedures
Blood samples collection and serum preparation
Whole blood samples from 200 camels were collected through jugular venipuncture using well labelled plain vacutainers tubes (5mL). The tubes were kept in ice box and then transported immediately to the Veterinary Laboratory of Somali National University. Serum samples were prepared per methods reported by Jesse et al. (2018) briefly, whole blood samples were centrifuged for 5 minutes at 3000× g. The harvested sera were then poured into a new 1.5 micro-centrifuge tube (Eppendorf) and stored until used for CATT.
Serological testing
Serological test for the detection of antibodies against T. evansi using Card Agglutination Test for Trypanosomiasis (CATT) (Institute of Tropical Medicine ANTWERP, Belgium) was performed per manufacturers recommendation. CATT is a direct and rapid agglutination test used for detection of T. evansi. Briefly, serum samples were diluted up to 1:4 with buffer. Then aliquot 25 μL of diluted serum was pipetted on to a plastic-coated test card, followed by the addition of one drop of CATT reagent. The reaction mixture was spread out gently by using a clean stirring rod and allowed to react on the card by manually rotating the card for 5 minutes. Blue granular agglutinations indicate a positive reaction visible to the naked eye.
Questionnaire
Data regarding the characteristics of individual camels, including age, sex, body condition and location of the farm were obtained using a well-structured questionnaire.
Data analysis
Analysis of data to determine the seroprevalence of T. evansi in camels was carried out using SPSS version 20. 0. Chi- square test was used to determine association between age, sex, body condition, location and the seroprevalence of T. evansi. The results were considered statistically significant at P<0.05 at 95% confidence level.
RESULTS
The results of this study showed that out of the 200 blood samples collected and examined, 129 (65%) samples were seropositive for T. evansi. Among the six farms sampled, Cagarey showed high seropositive rate 25 (80%) followed by Goorsan 27 (75%), Mandeq 16 (61%), Albaraka 36 (60%), Sahan 12 (55%) and the lowest being Alkhalil with seroprevalence of 13 (52%) (Table 1).
Risk factor analysis
Analysis of risk factors using chi-square test at P<0.05 and 95% confidence level showed that there was no statistically significant association between age, sex, body condition and farm location with the seroprevalence of T. evansi in camels. However, high seropositive rate was observed in camels >3years of age (67%) than those between ages 1-2 years (54%) p> 0.05 (Table 1). Similarly, seropositive rate to T. evansi was observed more in female camels (66%) 121 than males (44%) 8 p > 0.05 (Table 1). Furthermore, seropositive rate was observed more in camels with normal body condition (65%) 121 than those with poor body conditions (50%) 16, P> 0.05.
DISCUSSION
Trypanosoma evansi, the most pathogenic and widely distributed animal trypanosome, is one of the major causes of economic losses among domestic livestock farmers in Africa, Asia, Central and South America and Europe (Salim et al., 2011). In semi-arid zones of Africa such as Somalia, Ethiopia, Kenya, Sudan, French West Africa, Nigeria and Chad, camels are the worst hit animal species by T. evansi (Salim et al., 2011). Although, trypanosomiasis caused by T. evansi is known to affect mostly animals, cases of human infection have been reported in India among a minority population of humans that carry defective form of APOL1 protein (Joshi et al., 2005; Acosta et al., 2016). Thus, indicating the zoonotic potentials of T. evansi. In this study, the overall seroprevalence of T. evansi in the sampled camels using CATT agglutination test was 65% (129/200). Similarly farm level seroprevalence showed that 80% of the camels in Cagarey farm were seropositive to antibodies against T. evansi, while Alkhalil farm had the lowest seroprevalence (52%) (Table 1). The difference in prevalence between farm was not statistically significant (P =0.1). The variations in prevalence of camel trypanosomiasis reported from these farms could be due to differences in the management system, vector density; control interventions practiced and lack of awareness by animal owners about the severity and economic impact of the disease. The results of this study were not in agreement with the findings of previous study conducted in Somaliland where Salah et al. (2019) reported a prevalence 26.4% out of 2757 blood samples collected from Camels. The study also showed that the seroprevalence of T. evansi was high in camels with poor body condition (39.5%) than those with normal or good body condition (18.4%). While in this study, camels with good body conditions (65%) had higher seroprevalence than those with poor body conditions (50%). This could possibly be due to sampling large proportion of camels with apparently good body condition score (this study), sensitivity and specificity of the detection methods and the season when samples were collected as well as farm management practice. The findings of this study also contrasted the findings of Hakeem (2019) where a seroprevalence of 91 (29.9%) was reported among camels in Sudan. The results on age as a potential risk factor is however in agreement with our findings where high seropositive rate was observed among camels greater than 3 years of age. This could be because of prolonged exposure to the vectors of T. evansi compared to young animals which had less exposure. Similarly, in another study conducted in Sudan, Bala et al. (2018) reported a relatively low prevalence of 2.9% from blood smears of camels. The seroprevalence of T. evansi reported in this study is also higher than those previously reported in Ethiopia (18.2% and 21%), Saudi Arabia (13.2%), Pakistan (21%) Tanzania (8.3%), Sudan (33%) and Kenya (28%) (Bogale et al., 2012; Zeleke and Bekele, 2001; Hussain, 1991; Shah et al., 2004; Njiru et al., 2002; Elamin et al., 1998; Swai et al., 2011). The disparity observed could be because of the difference in sample size, detection method, climatic conditions, farm management practice, endemicity of the disease or vector and exposure to other stressors that may interfere with the immunity of the camels.
Table 1: Seroprevalence of T. evansi in camels based on age, sex, body condition and farm location.
| S. No. | Variables | Number tested | Number positive | Seroprevalence (%) |
| 1 | Farms | |||
| Goorsan | 36 | 27 | 75 | |
| Cagarey | 31 | 25 | 80 | |
| Alkhalil | 25 | 13 | 52 | |
| Albaraka | 60 | 36 | 60 | |
| Mandeq | 26 | 16 | 61 | |
| Sahan | 22 | 12 | 55 | |
| 2 | Age | |||
| 1- 2 yrs | 35 | 19 | 54 | |
| >3 yrs | 165 | 110 | 67 | |
| 3 | Sex | |||
| Male | 18 | 8 | 44 | |
| Female | 182 | 121 | 66 | |
| 4 | Body condition | |||
| Normal body condition | 184 | 121 | 65 | |
| Poor body condition | 16 | 8 | 50 |
In this study, camels >3 years of age have higher infection rate even though the result is not statistically significant (P>0.05). This agrees with the findings of Delafasse and Doutoum (2004) and Bhutto et al. (2010) where high antibodies against T. evansi was reported in camels greater than 3 years of age than those between 1-2 years of age. The result was also in consonant with the report of Atarhouch et al. (2003) where it was revealed that the infection rate of T. evansi increase with increase in age of up to maximum of 7-10 years. This may be because calves are usually not allowed to graze in the field with the adults and are not exposed to long distance grazing where they may encounter the vectors. Furthermore, in Mauritania, Jacquiet et al. (1994) reported that young calves below one year of age were free of Trypanosoma evansi infection. However, Pathak and Khanna (1995) reported that all camels were equally susceptible to trypanosome infection regardless of breed and age.
In this study, the seroprevalence of T. evansi in female camels was higher than in males. This agrees with the findings of Bhutto et al. (2010) where high seroprevalence of T. evansi was reported in females than in males. Shah et al. (2004) also reported high prevalence of T. evansi in females. On the other hand, Bogale et al. (2012) reported high seroprevalence in males than in females. He attributed his findings to the fact that male camels were exposed to much stress than the females.
CONCLUSION
The result of this study affirmed the occurrence of T. evansi among camels in Benadir region of Somalia and it provides useful baseline data on camel trypanosomiasis.
ACKNOWLEDGEMENT
The authors wish to thank the management of Faculty of Veterinary Medicine and Animal Husbandry, Somali National University for technical and financial support.
Authors Contribution
MAM conceived and design the research study, AAM and HIA collected the data and performed the experiment, MAM and AAB analyze the data and drafted the manuscript. All authors approved the final draft of the manuscript.
Conflict of interest
None to declare
REFERENCES





