Advances in Animal and Veterinary Sciences
Research Article
Clinical Picture and Haemogram Profile Associated with Theileria annulata Infection in Cattle before and after Therapeutic Intervention
Sarah G. Yousef1, Farouk A. El Balkemy1, Yousry A. El-Shazly2, Hend M. El Damaty1*
1Department of Animal Medicine, Infectious Diseases, Faculty of Veterinary Medicine, Zagazig University, 44511, Zagazig, Sharkia, Egypt; 2Veterinary Hospital, Faculty of Veterinary Medicine, Zagazig University, 44511, Zagazig, Sharkia, Egypt.
Abstract | Bovine theileriosis listed one of the tick-borne diseases of crucial concern worldwide.. The objectives of this study were to investigate the occurrence of tropical theileriosis in a farm of cattle demonstrating acute manifestations by Giemsa stained blood smears and Tams1gene based polymerase chain reaction (Tams-1 PCR). Moreover, to follow up the treatment with buparvaquone and long-acting oxytetracycline on the clinical, parasitological and hematological profile of infected cattle. Blood samples were collected from cattle (n=25) suspecting Theileria annulata (T. annulata) infection and healthy ones (n = 10; clinically and parasitologically free) in Sharkia Governorate Egypt, during July-August 2019. Fever (40-41°C), superficial lymph nodes enlargement, corneal opacity, and cases of non-specific abortion were the most manifestations recognized. The microscopic examination of blood smears (n=25) showed intracellular signet ring piroplasms which, confirmed using Tams1gene based PCR that identified Theileria parasites as T. annulata. Haemogram revealed normocytic normochromic anemia, meanwhile, the total leukocyte count (TLC) and lymphocytes significantly increased (P ≤0. 05) compared to the control ones using T-test. The cure rate in all treated cattle was (88%; 22 / 25) two weeks after the treatment. A rapid decrease in parasitemia in adults and young cattle to 0.5%, and 7%, respectively one week after treatment, and a significant increase in hematocrit percent and erythrocyte count, as well as macrocytic hypochromic anemia, was recorded two weeks from the beginning of the therapy. In conclusion, the response of Infection with T. annulata plays a vital role in occurrence of anemia and in changing blood parameters. Buparvaquone and oxytetracycline are indicated in the treatment of acute theileriosis together with iron supplements.
Keywords | Buparvaquone, Cattle, Haemogram, PCR, Theileria annulata
Received | September 10, 2019; Accepted | February 17, 2020; Published | March 03, 2020
*Correspondence | Dr. Hend El Damaty, Assistant professor at the Department of Animal Medicine, Infectious Diseases, Faculty of Veterinary Medicine, Zagazig University, 44511-Zagazig, Sharkia, Egypt; Email: hendvet11@yahoo.com, hmsaad @zu.edu.eg
Citation | Yousef SG, El-Balkemy FA, El-Shazly YA, El-Damaty HM (2020). Clinical picture and haemogram profile associated with Theileria annulata infection in cattle before and after therapeutic intervention. Adv. Anim. Vet. Sci. 8(3): 290-296.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.3.290.296
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Yousef et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Bovine theileriosis listed one of the tick-borne diseases that caused high economic losses in tropical and subtropical areas caused by many species of Theileria, the most critical one is Theileria annulata (T. annulata) (Mukhebi et al., 1992). Tropical theileriosis took into consideration as one of the negative limitations for livestock production in Egypt (Al–Gaabary, 1995). Clinical findings and microscopic examination of Giemsa stained thin blood smears are still the most common and economically method used for the diagnosis of acute theileriosis (Aktas et al., 2006). However, the carrier cases and morphological similarity of Theileria sp. piroplasm are limitations of the above mentioned diagnostic tools (Gul et al., 2015). Thus, Molecular techniques have greater sensitivity and specificity over the direct microscopy for differentiation among Theilreia sp. using specific primers (Bishop, 1992). Gene encodes a major piroplasm merozoite surface antigen of T. annulata has an advantage as it is conserved in all T. annulata stocks with a lower chance of cross-reactivity with other species of Theileria (Katzer et al., 1998). The main features of tropical theileriosis are anemia, lymphoproliferative disorders together with suppressed immune status. The extent of parasitemia is a good indicator of anemia, as the magnitude of anemia, related to increasing parasitemia (Boulter and Hall, 1999). Until now, buparvaquone is the best theilericidal drug which acts against both piroplasms and schizonts, yet it has a poor action against peracute and advanced cases (Osman and Al-Gaabary, 2007). The adequacy of buparvaquone in the treatment of acute theileriosis has fluctuated from (88.7 to 100%) (Hashemi-Fesharki, 1991; Qayyam et al., 2010), respectively. Long-acting antibiotics are requested significantly to keep away from secondary infections, in particular, respiratory ones, supportive drugs are also required to decrease the impact of anemia and oxidative stress. Hence, the objectives of this study were to investigate the occurrence of tropical theileriosis in a farm of cattle, demonstrating acute manifestations by Giemsa stained blood smears and Tams1gene PCR. Moreover, to follow up the treatment with buparvaquone and long-acting oxytetracycline on the clinical, parasitological, and hematological profile of infected cattle.
MATERIALS AND METHODS
This study conducted on naturally infected animals following the guidelines of the Zagazig University IACUC Committee, approval number ZU-IACUC/2/F/25/2019.
Animals
During summer 2019, a total of 25 crossbred cattle, 12 adults (3-7 years) of which 11 females and one male and 13 young (6-10 months) of which 4 females and 9 males included in this study. Animals reared in a smallholder farm under the semi-intensive system at Diarb Negm, Sharkia Governorate, Egypt, and clinically examined according to the protocol of Constable et al. (2017). In addition to the clinical examination of mucous membranes, eyes and nosrtil and the palpation of superficial lymph nodes during each visit, rectal temperature of each examined animal was taken to assess the clinical improvement.Investigated animals selected after the owner’s complaints including, abortion, high fever, and enlargement of lymph nodes, corneal opacity, lacrimation, anorexia, ocular, and nasal discharge with different degrees of ticks infestation, they previously have not taken any acaricides. They were subjected to parasitological and hematological examination after consent from the farm owner to used his animals for our study.
Moreover, ten cattle from another farm with no history of tick infestations and regular use of acaricides and under the nearly same conditions were used as a control during the hematological examination. These cows were examined for any abnormal clinical symptoms and external parasites like ticks and lice. These animals examined for any internal parasites using different techniques such as flotation, sedimentation, and Barmen’s techniques, as well as examining stained blood films to confirm haemoparasites absence.
Sampling and blood parameters
Blood smears were collected from puncture of the ear veins of clinically infected (n =25) and control animals (n=10). Blood samples were collected from the jugular vein on EDTA and divided into two portions; one for the examination of the hematological parameters using the Auto hematology analyzer (Rayto, RT-7200 Germany) (Fieldman et al., 2000) and the others preserved at −20°C and used for DNA extraction. Those samples kept at 4 °C in an icebox and transported without delay to the laboratory of the Department of Animal Medicine, Faculty of Veterinary Medicine, Zagazig University, Egypt.
Parasitological examination
The methanol fixed, Giemsa stained thin blood smears were examined at 1000× magnification (Moretti et al., 2010) using a Light ordinary microscope for detection of Theileria piroplasm. Parasitemia assessed as the percentage of infected erythrocytes after examined five different microscopic fields (1,000 erythrocytes)(Neelam et al., 2017).
DNA extraction
200 µL blood from clinically infected animals (n=25) and control ones (n =10) were exposed for genomic DNA extraction using a commercial QIAamp DNA blood Kit (Qiagen, Ltd, UK, Cat. No. 51304,) according to the manufacturer instruction.
PCR amplification
A 785bp fragment of the T. annulata 30 KD, a major merozoite surface antigen gene, amplified using Tams-1primers; Tams1F (5′- ATG CTG CAA ATG AGG AT-3′) and Tspms1R (5′- GGA CTG ATG AGA AGA CGA TGA G -3′) (Kirvar et al., 2000). For control animals, for detection of Theileria sp. the primers’ sequence were F 5’AGTTTCTGACCTATCAG3’ and R 5’ TTGCCTTAAACTTCCTTG3’ of 18S rRNA gene (370 bp) (D’Oliveira et al., 1995).
A mixture (25 μL) containing 1 μL of each primer (20 pmol), 6 μL of template DNA, 12.5 μL of Emerald Amp GT PCR master mix (2x premix) (Takara) and 4.5 μL of PCR grade water prepared. The amplification done in a thermal cycler (TECHNE TC–312), as follows ; initial denaturation at 94 °C / 5 min, 35 cycles of denaturation at 94 °C / 30 S, annealing at 55 °C / 40 S, extension at 72 °C / 55 S and a final extension cycle at 72°C / 10 min. 10 μL of each. The amplified products were run on a 1% agarose gel (Applichem GmbH; Darmstadt, Germany) along with negative control samples and the positive control, with a template of DNA from a reference strain (T. annulata AB917285) was also amplified (Elsify et al., 2015). Then stained using 0.5 μg/ mL ethidium bromide and the bands photographed and their size determined using the computer software supplied Gel Doc UV gel documentation system (AlphaInno-tech; SanLeandro, CA, USA). This occurred in the Biotechnology Unit, Animal Health Research Institute, Dokki, Giza, Egypt.
Treatment intervention
Buparvaquone (BVP Ltd.Co. Kerry IRLAND) (2.5mg/kg Bodyweight (BW), I/M) used as a single dose and repeated after 48 h if no clinical improvement observed. In addition to oxytetracycline long-acting 20% (Noor Brook) (20 mg/kg BW, I/M) given at 3 doses with 48 h intervals to keep the animals away from secondary pulmonary troubles. The iron supply used, 10 mL IM for adults, and 5 mL for younger animals three times weekly. Ticks control achieved through the spraying the infected animals and the farm two times, 2 weeks apart to all infected animals (n =25) in the farm, and following up the treated animals clinically and upon the parasitological examination done on days 1, 3, 7 and 14 post-treatment in step with Dolan et al. (1992) and Qayyam et al. (2010).
Statistical analysis
Statistics related to hematological parameters were subjected to the T-test using the SPSS statistic software program (SPSS version 22 Inc, USA) for comparison between the control and diseased animals. All values expressed as means and standard errors and (P ≤0. 05) statistically considered significant.
RESULTS
Clinical, parasitologial and hematolgial findings before the treatment
Cattle infected with T. annulata (n=25) suffered clinically from inappetence, enlargement of superficial lymph nodes , fever with mean temperature values (40.1 and 40.2°C) in adult and young cattle, respectively, pale mucous membrane, corneal opacity, lacrimation, anorexia, nasal discharge, and abortion that recorded in (9 / 25; 36%) infected cows (Table 1).
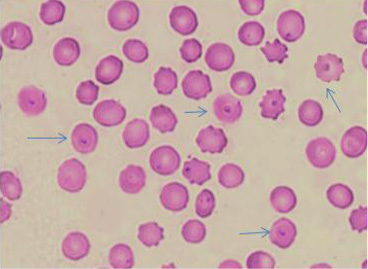
The microscopic examination of blood smears showed intracellular signet ring piroplasms (Figure 1). The percentage of parasitemia in 5 random microscopic fields was (16.1 and 34.9%) in adult cattle, and young calves, respectively (Table 2). The molecular results confirmed the results of blood smears of infected cattle (n=25) using Tams1gene based PCR and identified Theileria parasites as T. annulata (Figure 2). The control group tested by PCR and blood samples (n =10) were negative using general primer targeting 18S rRNA gene (370 bp) for the detection of Theileria sp.
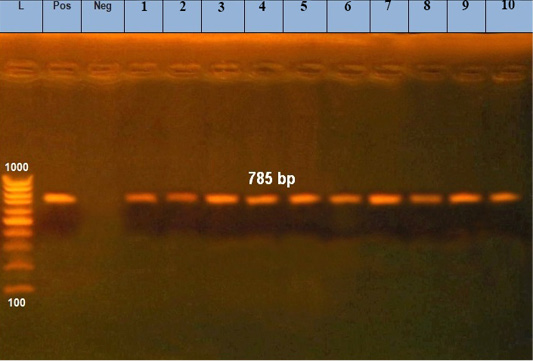
Figure 2: Amplified products of 30 KD a major merozoite surface antigen gene of rDNA for T. annulata. Lane L, molecular size marker (100 base-pair ladder); lanes1: 10 positive samples at 785bp; Lane Neg: negative control, Lane Pos: positive control.
Table 1: Observed clinical symptoms in Theileria annulata infected cattle.
| Clinical picture | Number of infected / diseased cattle | % |
| Fever | 25 / 25 | 100 |
| Enlarged superficial lymph nodes | 25 / 25 | 100 |
| Anorexia | 25 / 25 | 100 |
| Corneal opacity | 5 /25 | 20 |
| Pale mucous membrane | 18 / 25 | 72 |
| Nasal / ocular discharge | 3 / 25 | 12 |
| Abortion | 9 /25 | 36 |
Table 2: Displaying mean values of temperature and the degree of parasitemia in young and adult cattle.
| Days of treatment | Mean values of temperature (˚C) | Parasitemia (%) | ||
| Young cattle | Adult cattle | Young cattle | Adult cattle | |
| Zero day | 40.2 | 40.1 | 34.9 | 16.1 |
| 1st day | 39.7 | 39.1 | 12.5 | 3.35 |
|
3rd day |
39.2 | 39 | 7.2 | 1.6 |
|
7th day |
39.1 | 38.8 | 7 | 0.5 |
|
14th day |
39.1 | 38.7 | 0.6 | 0.3 |
As shown in Table 3, a highly significant decrease (P ˂0. 01) in the erythrocyte count, hematocrit (HCT) and haemoglobin (Hb) percentages was reported in T. annulata infected cattle compared to the control ones. While, a substantial increase (P ˂0. 05) in mean corpuscular hemoglobin (MCH), total leukocyte count (TLC) and lymphocyte count as well as a non-significant change is observed in mean corpuscular volume (MCV) and mean corpuscular hemoglobin concentration (MCHC).
Table 3: Haemogram of parasitologically normal and T. annulata infected cattle before and after treatment (mean values ± SE).
| Variable | Control (n=10) |
T. annulata infected cattle (n =22) |
T. annulata treated cattle (n =22) |
|
TLC1 (x 10³ /μl) |
7.39 ± 0.76 | 10.06 ± 0.58* | 8.81 ± 0.55 |
| Lymphocyte (x 10³ /μl) | 4.76 ± 0.68 | 6.68 ± 0.42* | 5.4 ± 0.35* |
|
TEC2 (x 106/ μl) |
6.55 ± 0.62 | 3.7 ± 0.12** | 4.06 ± 0.11* |
|
Hb3 (g / dl) |
10.91 ± 0.92 | 7.12 ± 0.19** | 7.2 ± 0.16 |
|
MCHC4 (%) |
34.1 ± 3.36 | 37.69 ±1.23 | 33.58 ± 0.76* |
|
MCH5 (pg) |
17.16 ± 1.25 | 19.41 ± 0.4* | 17.98 ± 0.31* |
|
MCV6 (fl) |
51.11 ± 1.04 | 51.94 ± 0.75 | 53.78 ± 0.69** |
|
HCT7 (%) |
33.97 ± 3.69 | 19.33 ± 0.82** | 21.85 ± 0.66* |
1 total leukocyte count; 2 total erythrocyte count; 3 haemoglobin; 4 mean corpuscular hemoglobin concentration; 5 mean corpuscular hemoglobin; 6 mean corpuscular volume; 7 hematocrit percent. * P ≤0. 05;** P ≤0. 01
Clinical, parasitologial and hematolgial findings after treatment
Cattle infected with T. annulata followed up clinically and upon the parasitological examination after treatment on days zero, 1, 3, 7, and 14. The therapeutic setup encompasses buparvaquone with oxytetracycline long-performing and iron supply. A good response of adult animals (n= 8/25) observed one day after the first dosing of therapy, and the mean temperature decreased to (39.1 °C) while, cows (n=2/25) suffered from pyrexia and required 2nd dose of buparvaquone 48 h later. Such cows appeared normal clinically at the end of the 1st week with mean temperature (38.8°C), meanwhile, the temperature begun to decline in young calves (n=12/25), 48 h after the 2nd doses of buparvaquone and return normal at the end of the 1st week with mean temperature (39.1°C) as shown in Table 2. Nasal and ocular discharges cured, and the inappetence restored before the end of the first week. Corneal opacity disappeared, the lymph nodes normally palpated, and conjunctival mucous membranes became pale rosy red two weeks after the treatment. The cure rate in animals under the study was (88%; 22/25) two weeks after the treatment as three (two adult cows and one young calf) died 2-3 days during treatment and not followed either hematologically or upon the parasitological examination.
A rapid drop in parasitemia from16.1 to 0.5% observed in adult cattle (n= 10/25) at the end of the 1st week. Meanwhile, a gradual reduction in parasitemia in young calves (n=12/25) from 34.9% to (7 and 0.6%) at the end of the 1st and 2nd weeks, respectively (Table 2).
The complete blood picture representing TEC, MCV, and HCT increased significantly, fourteen days from the initiation of the treatment, while Hb percentage increased but non-significantly (P >0. 05), a significant decrease in lymphocyte, MCHC, and MCH, while TLC decreased but not significantly.
DISCUSSION
In Egypt, tropical theileriosis has taken into consideration as one of the most negative limitations for livestock production (Al–Gaabary, 1995). The most common symptoms recorded in this study were fever, anorexia, enlargement of superficial lymph nodes, lacrimation, and corneal opacity, this comes with an agreement with previous studies (Abdel Rady et al., 2010; Mahmmod et al., 2011; El-Dakhly et al., 2018).
The parasitemia in adult cattle and young calves before treatment was 16.1 and 34.9%, respectively. This agreed with Modi (2013) who reported that parasitemia ranged from less than 2% up to 40% in his study on theileriosis in crossbred cattle . Haemogram of examined animals confirmed a highly significant reduction in the erythrocyte indices (HCT and Hb %, and TEC). The same conclusion reported in other studies in cattle (Alam and Naser, 2011; Ramin et al., 2011). Normocytic normochromic anemia attributed to erythrophagocytosis of damaged RBCs, which evoked due to the induction of cytokine storm (Singh et al., 2001). However, leucogram revealed significant lymphocytosis and leukocytosis, which may be due to the synchronous division of infected lymphoblasts with schizonts (Demessie and Derso, 2015). Our findings consequences with the results of Abdul-Husin et al. (2016), while inconsistent with those reported by Omer et al. (2002), who observed tremendous leucopenia in their hematological evaluation in cattle. On the other hand, (Ramin et al., 2011) didn’t find any significant difference in either TLC or lymphocyte count between diseased and control groups. The variation of leucogram pictures may be associated with the phase of the disease and the extent of the infection. (El-Deeb and Younis, 2009).
After therapeutic medication, the clinical and parasitological recovery of adult cattle was quicker than the young calves. The difference between adults and young animals in response to therapy may be explained by the fact that newborn calves have innate resistance acquired by maternal immunity, which declines gradually leaving the animal with greater susceptibility from about 6 months to 3 years (Brown, 1990). This agreed with El-Masry et al. (2006) and Al-Hosary et al. (2018) who concluded that cattle under one year of age are more susceptible to infection than adults, whereas, three animals (two adult cows and one young calf) died 2-3 days throughout the treatment plan. This may be due to the immune response of these animals, the severity of the anemia, and the failure to overcome respiratory complications. This finding is concurs with Muraguri et al. (2006) and Kachhawa et al. (2016). A sharp decrease in the proportion of parasitemia to less than 0.5% in adult cattle after the 1st dosing of medication whereas, the gradual reduction ascertained in younger calves, this might explain the distinction of mean temperature values within the 1st and 3rd-day post-treatment in adult and younger animals. Parasitemia reached below 1% by the end of the 1st week in adult cattle and 2nd week in young calves. These results came in accordance with Modi (2013) who treated 12 cattle clinically infected with theileriosis by buparvaquone (2.5 mg/kg BW) and the treated cows were parasitologically negative two weeks post-treatment. Meanwhile, Neelam et al. (2017), recorded that the parasitemia was 0.42±0.04 and all treated animals were free from piroplasms three days post-treatment regardless of their age.
In this study, the normal state of the infected animals started to retain fourteen days after treatment, with the indications of hematological regeneration as anemia that become macrocytic hypochromic, with a significant increase in erythrocyte count, and HCT % but, still lower than the healthy control ones. The same conclusion reported by other studies (Altug et al., 2014; Kachhawa et al., 2016). Meanwhile, Neelam et al. (2017) reported a non-significant change in post-treatment hematological parameters which may be due to the recruitment of cattle for treatment with mild form of the disease or may be due to short follow-up after treatment was not sufficient to achieve a significant change in hematological parameters. The results revealed a significant reduction in lymphocytes and a non-significant decrease in TLC, but still higher than the healthy control cattle. Similarly, Abdul-Husin et al. (2016), reported a significant reduction in lymphocytes after buparvaquone and oxytetracycline treatment. The cure rate in treated cattle two weeks after treatment was (88%; 22/25), these findings are almost similar to those previously reported (Muhammad et al., 1999; Al-Hosary et al., 2010) with a recovery rate of 93 and 81.81%, respectively. This may be related to the early interference with the treatment as (Osman and Al-Gaabary, 2007) concluded that early diagnosis of the disease increases the recovery rates.
CONCLUSION
The response of T. annulata infected adult cattle to treatment was faster than young calves. Infection with T. annulata plays a vital role in occurrence of anemia and in changing blood parameters. Buparvaquone and oxytetracycline are indicated in the treatment of acute theileriosis together with iron supplements. This study documented that animals under small scale production need increasing attention from agriculture authority to put the proper acaricidal application programs.
ACKNOWLEDGMENTS
The authors would like to thank the cattle owner who provided access to the data about its private farm. The current research was financially supported by the necessary facilities of the Department of Animal Medicine, Faculty of Veterinary Medicine, Zagazig University. The authors are grateful to Dr. Yasser Mahmmod, Dept. of Animal Medicine, Faculty of Veterinary Medicine, Zagazig University, for his support.
Author’s Contribution
FAE and HME contributed to the proposal of the manuscript; SGY and YAE collected the samples needed for the experiment, carried out the experimental procedure; SGY and HME analyze the data, drafted and reviewed the manuscript; All authors have revised and approved the manuscript.
Conflicts of interest
No conflict of interest noted. No financial implication.
REFERENCES