Advances in Animal and Veterinary Sciences
Research Article
In vivo Efficacy of Biophytum petersianum on Haemonchus contortus in Goats
Priyo Sambodo1, Joko Prastowo2, Kurniasih Kurniasih3, Wida Wahidah Mubarokah4, Soedarmanto Indarjulianto5*
1Department of Animal Science Papua University, Jalan Gunung Salju, Amban, Manokwari Papua Barat, 98314, Indonesia; 2Department of Parasitology, Faculty of Veterinary, Universitas Gadjah Mada, Bulaksumur, Caturtunggal, Depok, Sleman, Daerah Istimewa Yogyakarta 55281, Indonesia; 3Department of Pathology, Faculty of Veterinary, Universitas Gadjah Mada, Bulaksumur, Caturtunggal, Depok, Sleman, Daerah Istimewa Yogyakarta 55281, Indonesia; 4Department of Animal Health, Agriculture Extension College, The Polytechnique of Agricultural Development Yogyakarta Magelang, Jalan Magelang-Kopeng Km 7 Purwosari, Tegalrejo, Magelang, Jawa Tengah, 56192, Indonesia; 5Department of Internal Medicine, Faculty of Veterinary, Universitas Gadjah Mada, Bulaksumur, Caturtunggal, Depok, Sleman, Daerah Istimewa Yogyakarta 55281, Indonesia.
Abstract | Haemonchosis is a common and severe disease caused by the infection of worms Haemonchus sp. Kebar grass (Biophytum petersianum) known as a plant from Papua Indonesia that contains tannin compounds and has potential anthelmintic activity. The present study was carried out to determine the influence of Kebar grass infusion on H. contortus under in vivo conditions. A total of 15 female goats at 6-8 months old were divided into 5 groups, and each consisted of 3 goats. All groups were infected 1000 infective larvae orally every week for 4 weeks. Three groups were given Kebar grass infusion a day at a dose of 2 mg/mL, 4 mg/mL and 6 mg/mL respectively for 7 days at the 6th week. Group 4 was given Albendazole at a dose of 3.8 mg/kg body weight, and group 5 was not given treatment. Clinical examinations, hematological and EPG were carried out every week. All goats were autopsied and analyzed for pathology at the seventh week. Quantitative data to perform statistical analysis, as well as the results of physical examination and histopathology were analyzed descriptively. Body weight measurements in vivo as the mean, Body Condition Scores (BCS), and FAMACHA showed an increase. RBC, Hb, PCV and total protein values increased. The FECR value of the treatment group was higher than the negative control group. During the autopsy, both carcass and visceral organs were colourless, slight of subcutaneous fat, and small intestine haemorrhage and ascites in the abdominal cavity. Many nodules with diameters between 1.5-2.0 mm were observed in the abomasal mucosa. Histopathological changes in the control group were infiltration of inflammatory cells, congestion and the presence of worm pieces. Based on results obtained in this line of research, it is therefore concluded that Kebar grass infusion is anthelmintic against H. contortus under in vivo conditions.
Keywords | Anthelmintic, Haemonchus contortus, Goats, Kebar grass
Received | October 07, 2019; Accepted | February 17, 2020; Published | March 05, 2020
*Correspondence | Sudarmanto Indarjulianto, Department of Internal Medicine, Faculty of Veterinary, Universitas Gadjah Mada, Bulaksumur, Caturtunggal, Depok, Sleman, Daerah Istimewa Yogyakarta 55281, Indonesia; Email: indarjulianto@ugm.ac.id
Citation | Sambodo P, Prastowo J, Kurniasih K, Mubarokah WW, Indarjulianto S (2020). In vivo efficacy of Biophytum petersianum on Haemonchus contortus in goats. Adv. Anim. Vet. Sci. 8(3): 238-244.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.3.238.244
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Sambodo et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
introduction
Haemonchosis is one of the major parasitic diseases in goats in Indonesia. The biggest economic losses due to haemonchosis occur in sub-tropical and tropical regions which are mainly due to mortality, decreased production, stunted growth and low body weight (Mengist et al., 2014). In fact, West Papua could be a potential development area for goat livestock. Based on data from the Department of Animal Husbandry of West Papua Province (2016), the number of goats in West Papua Province has continued to increase by more than 77% within 4 years. One obstacle in developing the livestock business is the high incidence of helminthiasis. With regard to this issue, Purwaningsih et al. (2017), stated that the incidence of gastrointestinal worm infestations in etawa-bred goats that were kept semi-intensively in Amban District, Manokwari was 100% (n: 32). At present, resistance to nematode populations has been detected in all livestock species, such as sheep, goats, cattle and horses (Kaplan, 2004). From actual cases appear, Kebar grass (Biophytum petersianum Klotzsch) developed as one of Indonesia’s endemic plants used as medicinal plant. The plant tannin content is 1.1% (Santoso et al., 2006) and condensed tannins are 0.28% (Sambodo, 2018). As well, Widayati and Nurhayati (2013), reported that at 60% dose of Biophytum petersianum Klotzsch is able to eradicate adult worm called Ascaridia galli in 7 hours which is used as sample. On the other hand, 60% dose infusion of Biophytum petersianum works independently to also execute those adults worms in 7.5 hours under in vivo condition. From such observations, it can be argued that the Kebar grass has potential as an anthelmintic of H. contortus worms under in vivo conditions.
Material and Method
Ethical Clearance
The entire research processes have received ethical eligibility from the Ethics Commission of the Gadjah Mada University in Yogyakarta (Certificate No.: 00116/04/LPPT/IX/2017).
Collection and Biophytum petersianum Infusion
Biophytum petersianum was obtained from the Central Kebar District (located at 133°03”25.8’ East Longitude and 00°48”31,1’ South Latitude with an altitude of 590 m asl) Tambrauw Regency, West Papua Province. First of all, some Kebar grass is aired, thus the Kebar grass is dried and stored until it is used for the extraction process (Sambodo et al., 2012).
The kebar grass is dried and finely chopped. A total of 1 gram (1%), 2 gram (2%), 4 gram (4%), 6 gram (6%), 8 gram (8%) and 10 gram (10%) dried Kebar grass were put into the Erlenmeyer and soaked in 100 mL aqua destillata. Erlenmeyer flask was incubated in an oven at 90 oC for 15 minutes. Furthermore the solution is filtered using filter paper and stored in a refrigerator for 24 hours (Daryatmo et al., 2010).
Treatment on Experimental Goats
The treatment was carried out according to Eguale et al. (2007). Experimental animals were 15 female goats aged 6-8 months divided into 5 groups. Four weeks after adaptation, all groups were infected with 1000 oral infective larvae every week for 4 weeks. At an average EPG value of 3000 EPG, all groups were given treatment for 7 days as follows group 1 (2 mg/mL), group 2 (4 mg/mL), group 3 (6 mg/mL), group 4/positive control (3.8 mg/kg BW) and group 5/negative control (not treated). All research parameter checks were carried out on the 0th and 7th days.
Clinical Examination
Clinical examination included weighing and physical examination in forms of eye conjunctiva (FAMACHA) and BCS (Eguale et al., 2007; Attindehou et al., 2012).
Hematology
Blood collection was carried out on all goats through the jugular vein of 2 ml/ goat. The parameters analyzed included RBC, Hb, PCV and total protein (Eguale et al., 2007; Qamar and Maqbool, 2012).
Fecal Egg Count Reduction (FECR)
Fecal sample collection was performed directly from the rectum. The EPG calculation was carried out using the McMaster technique according to Coles et al. (1992). The percentage decrease in the number of worm eggs per gram of faeces or FECR was calculated by the following guidelines:
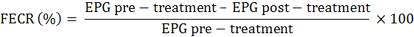
Postmortem examination
The necropsy technique was carried out mainly according to Somvanshi and Rao (2009), with several modifications. After physical examination, the goats are slaughtered by cutting the four neck veins. Move forward to perform the dissection to the dead goats. Examination of the carcass, visceral organ and intestine as well as the presence or absence of fluid in the abdominal cavity. Macroscopic examination of mucosal abomasum and tissue collection as histological preparations were made according to Muntiha (2001).
Data Analysis
The study adopted quantitative data to perform statistical analysis. The findings were presented as mean ± standard deviation. The difference from the effect of different treatments was analyzed by analysis of variance with SPSS 23 (IBM Corp). The correlation between physical examination and histopathological data were presented descriptively.
Results
Clinical Examination
In the current project, the mean body weight of goats increased post-treatment compared to pre-treatment (Table 1), although paired t-tests did not produce a significant difference (P>0.01) and the value of BCS has increased, yet it is still in the low range. The value of FAMACHA after treatment increased compared to pre-treatment, where treatment on group 3 experienced the greatest increase (Figure 1).
Table 1: Average weight of the treatment group.
| Group | Body weight | |
| Pre-treatment | Post-treatment | |
| Group 1 (2 mg/mL) |
12.67±1.15a |
13.50±1.00a |
| Group 2 (4 mg/mL) |
14.00±1.32a |
14.50±2.50a |
| Group 3 (6 mg/mL) |
13.00±1.00a |
13.67±1.53a |
| Average |
13.22±1.18a |
13.89±1.62a |
The same superscript in the same line are not significantly different (P>0.005).

Figure 1: FAMACHA value of the treatment group. (a) Pre-treatment (value 5) (b) post-treatment (value 3).
Table 2: Mean haematological values of pre-treatment and post-treatment.
| Group | Parameters | Pre-treatment | Post-treatment |
|
Control + (Albendazole 3.8 mg/kg BW) |
RBC (µL) |
2.23±0.38a |
2.25±0.31a |
| Hb (dL) |
9.00±0.82a |
9.30±0.60a |
|
| PCV (%) |
25.00±4.21a |
26.00±3.91a |
|
| Serum protein (g/dl) |
6.60±0.32a |
6.70±0.44a |
|
| Group 1 (2 mg/mL) | RBC (µL) |
1.92±0.84a |
2.11±0.45a |
| Hb (dL) |
8.00±2.53a |
7.30±2.61a |
|
| PCV (%) |
20.00±11.45a |
18.95±9.05a |
|
| Serum protein (g/dl) |
6.00±0.45a |
6.40±0.48a |
|
| Group 2 (4 mg/mL) | RBC (µL) |
2.27±0.75a |
2.75±0.88a |
| Hb (dL) |
8.00±1.58a |
9.00±1.56a |
|
| PCV (%) |
25.00±8.26a |
30.00±10.72a |
|
| Serum protein (g/dl) |
7.00±0.11a |
7.00±0.82a |
|
| Group 3 (6 mg/mL) | RBC (µL) |
1.91±0.11a |
2.67±0.49a |
| Hb (dL) |
7.00±3.27a |
9.00±1.17a |
|
| PCV (%) |
20.00±10.91a |
30.00±4.97a |
|
| Serum protein (g/dl) |
3.00±0.51a |
7.00±0.15a |
|
| Control - (saline water 0,62%) | RBC (µL) |
2.33±0.54a |
2.01±0.49a |
| Hb (dL) |
8.90±1.25a |
7.90±1.21a |
|
| PCV (%) |
23.30±3.79a |
19.80±3.56a |
|
| Serum protein (g/dl) |
6.90±0.93a |
5.00±2.03a |
The same superscript in the same line are not significantly different (P>0.005).
Hematology
RBC, Hb, PCV and total protein values post-treatment with Kebar grass infusion showed an increase compared to pre-treatment, where for all parameters, treatment group 3 experienced the greatest increase (Table 2).
FECR
At this stage, the average EPG for the 3 treatment groups was 2955 EPG. The average EPG pre and post treatment as well as the results of FECR calculations in each treatment group can be illustrated in Table 3.
Table 3: Average EPG and FECR treatment groups.
| Groups | EPG | FECR (%) | |
| Pre-treatment | Post-treatment | ||
| Control + (Albendazole 3.8 mg/kg BW) | 1583±575.18 | 0±0.00 | 100.00 |
| Group 1 (2 mg/mL) |
2217±125.83a |
0±0.00b |
100.00 |
| Group 2 (4 mg/mL) |
3650±2995.41a |
0±0.00b |
100.00 |
| Group 3 (6 mg/mL) |
3000±2133.66a |
17±28.87b |
99.44 |
| Control - (saline water 0.62%) | 1900±888.82 | 1450±390.51 | 23.68 |
a,b means with different superscripts in the line are significantly different (P<0.005).

Figure 2 Mucosa abomasum control and treatment group. (a) positive control; (b) treatment 1, nodule (arrow); (c) treatment 2; (d) treatment 3; (e) negative controls, nodules (arrow).
Postmortem Examination
Anatomical Pathology
In the present study, the carcass condition between the negative control group and the treatment group was not relatively different, the two carcasses were colorless and subcutaneous fat was highlighted in small amount, the visceral organs of the negative control group appeared paler and the small intestine in both groups showed hemorrhages. There was ascites fluid in the abdominal cavity, although the amount was more in the negative control group. In the abomasal mucosa the negative control group and treatment on group 1 had many nodules with a diameter between 1.5-2.0 mm (Figure 2).
Histology
Histopathological examination revealed abnormal conditions in both the control and treatment groups (Figures 3). In abomasum tissue, it was found inflammation infiltration, congestion, larvae 4 and adult worms.
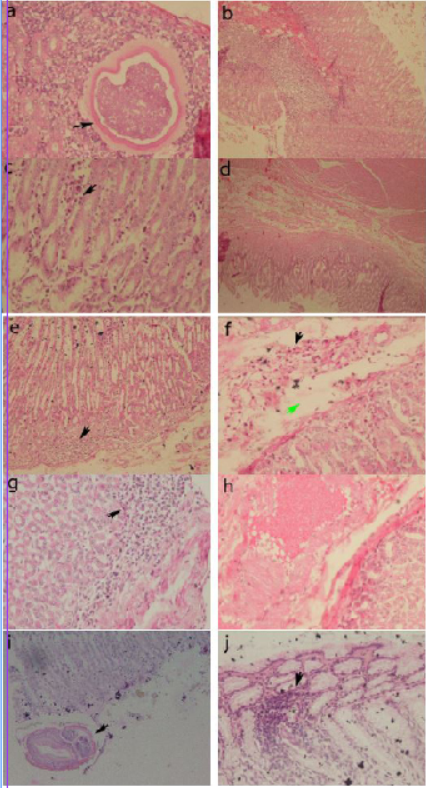
Figure 3: Histopathology of the abomasum control group. (a) positive control, larvae 4 in the submucosa (arrow) (40x); (b) positive control, congestion of blood vessels in the sub mucosa (20x); (c) group 1, infiltration of inflammatory cells in the sub mucosa (arrow) (40x); (d) group 1, edema in the sub mucosa (4x); (e) group 2, infiltration of inflammatory cells in the sub mucosa (arrow) (20x); (f) group 2, inflammatory cell infiltration (black arrow) and edema in the sub mucosa (green arrow) (40x); (g) group 3, infiltration of inflammatory cells in the sub mucosa (arrow) (40x); (h) group 3, congestion of sub mucosal blood vessels (40x); (i) negative control, cross section of adult worms in the mucosa (arrow) (20x); (j) negative control, infiltration of inflammatory cells in the sub mucosa (arrow) (40x).
Discussion
Clinical Examination
Aside from the experimental goats used at a young age of 6-8 months, the low body weight and BCS in this study were directly proportional to the low RBC value and total serum protein due to helminth infections. Regarding this issue, Eguale et al. (2007) reported, goats kept in animal husbandry and given dry feed; lose blood plasma and protein due to parasites which is would prevent goats to gaining weight.
The level of anemia can be roughly evaluated by looking at the color of the conjunctiva where the blood capillaries are on the surface, thus the color of the tissue reflects the color of the blood. In general, the value of FAMACHA in this study is in the normal range (3), that in such formulated category, the goats do not need to be given anthelmintics even though Vatta et al. (2001), reported that the goats in the FAMACHA 3, 4 and 5 categories were still mainly considered to be given anthelmintics, because the conjunctival color range is smaller in goats than sheep, and as a result, the application process of the FAMACHA system slightly can be difficult to apply. In addition, the sensitivity only reaches 67-69%.
Hematology
An increase in each parameter after treatment was remarkably considered to be due to the reduction or absence of adult worms in the treatment group, evidenced by the results of the necropsy abomasum of the treatment group that no longer found adult worms. The RBC value and total serum protein are lower than the reference according to Rasedee (1981). This decrease in RBC may be caused by the amount of blood losses during an infection. Each worm can suck 0.05 ml of blood per day (Urquhart et al., 1996). The decrease in total serum protein may be due to blood thinning due to abomasal bleeding caused by larval invasion (Qamar and Maqbool, 2012).
FECR
During the treatment, no poisoning effect was found in all experimental animals. In addition, at the time of necropsy, no adult worms were found in all abomasum from the positive treatment and control group. EPG values before and after treatment decreased significantly (P = 0.00). The results of this study are in accordance with previous studies. Iqbal et al. (2004), stated that a dose of 3 g / kg BW of water extract of Artemisia brefivolia was able to reduce the EPG value of sheep GI nematodes by 67.2% and was added by Tariq et al. (2009), that a dose of 2 gm / kg BW of water extract Artemisia absinthium was able to reduce the EPG value of sheep GI nematodes by 80.49%. Artemisia plants are rich in proanthocyanidins (condensed tannins) (Lutgen, 2018). Athanasiadou et al. (2001), reported that FEC and worms in the small intestine would decrease in number in sheep fed with pellets containing condensed tannins from Quebracho tree extracts. Sheep fed with Sainfoin straw containing dry condensed tannins decreased FEC (Paolini et al., 2003) and those fed Acacia karoo dried leaves decreased both FEC and the number of worms (Kahiya et al., 2003).
In this study, it was believed that the effects of condensed tannins occurred directly. The condensed tannins in the Kebar grass extract directly inhibit the hatching of worm eggs, where the EPG value drops significantly. In addition, condensed tannins also binds to protein, where under in vitro test there is a decrease in the number of protein bands and body surface damage in adult worms, and therefore, they become inactive and finally die.
Postmortem Examination
Anatomical Pathology
These results are mainly caused by H. contortus infestation. Haemonchus contortus suck blood which causes blood loss around the tissue make it looks colorless and resulting in decreased body weight . On the other hand, blood loss will drop blood albumin levels and cause fluid accumulation in the abdominal cavity. Both adult and fourth stage larvae suck blood and in addition, migration of adult and larvae cause haemorrhages into the abomasum.
Saminathan et al. (2015) suggested that in sheep infected with H. contortus has pale carcasses, little subcutaneous fat, yellowish fluid in the abdominal cavity, pale internal organs and small intestine congested, watery and petechiae hemorrhage and added by Estuningsih et al. (1996), that the surface of the abomasum infected with H. contortus showed changes in the form of lesions, inflammation due to worm bites. Edema of the folds of the omasum, hemorrhage of petechiae and the formation of nodules in the infected abomasum may be caused by the activity of the worm while sucking blood (Soulsby, 1982).
Histopatology
Attachment of the parasite with the abomasum mucosal causes histopathological changes and infection. Histopathological changes caused by H. contortus infection, such as the occurrence of a strong inflammatory response in the infected area characterized by cellular infiltration in the form of fibroblasts, macrophages, lymphocytes, plasma cells, mast cells, neutrophils and eosinophils (Balic et al., 2002; Ortolani et al., 2013; Saminathan et al., 2015).
Although many behaviors could be considered as abnormal in the treatment group, it was also believed that the major cause was due to the induction of previous haemonchosis. Although therapy has been conducted and no adult worms were found on the surface of the abomasum, the condition of goats tried to influence the healing process. The ability of the host to develop immunity and the level of resistance against GI nematodes depends on many factors, such as type, age, number of worms and nutrition (Stear and Murray, 1994; Hoste et al., 2005; Rowe et al., 2008). Pathology caused by parasites and host reactions to infecting parasites depend on the organ being attacked, depth of penetration, location and number of parasites (Taraschewski, 2000; Esmaeilnejad et al., 2012; de Oliveira et al., 2013) and added by Hoste et al. (2008), compared to sheep, goats develop fewer immune responses against nematodes, which in turn might affect the state of pathology and the ability to control infection.
Research on this issue, the presence of larvae (L4) in the abomasum tissue of the group given Albendazole, may be due to the low dose of the drug that contacts the parasite when it enters the body of the host. The metabolism of an anthelmintic in a host affects the anthelmintic concentration in the parasite (Lifschitz et al., 2017).
Conclusion
Based on clinical examination, hematological values, FECR values and postmortem examination in vivo, Kebar grass infusion is anthelmintic against H. contortus.
Acknowledgments
This study was supported by the Ministry of Research, Technology and Higher Education of the Republic of Indonesian through Domestic Graduate Scholarship (BPP-DN) program, grant number 1265.35/E4.4/2015.
Conflict of interest
The authors declare that they have no competing interest.
Authors Contribution
PS, JP, KK and SI the researchers. PS, JP, KK and SI developed the research concepts. PS and JP prepared H. contortus; PS, WWM and SI managed and examined goats during in vivo experiments and interpreted the results; PS and KK made histopathological preparations and interpreted the results; PS and WWM collected data and literature and prepared manuscripts. JP, KK and SI guided and corrected and complete the manuscript.
References





