Advances in Animal and Veterinary Sciences
Research Article
Isolation and Characterization of Bacteriophages against Escherichia coli Isolates from Chicken Farms
Nguyen Trong Ngu1*, Huynh Tan Loc1, Nguyen Thi Hong Nhan2, Pham Khanh Nguyen Huan3, Luu Huynh Anh2, Nguyen Hong Xuan4
1Department of Veterinary Medicine, College of Agriculture, Can Tho University, Can Tho City, Vietnam; 2Department of Animal Sciences, College of Agriculture, Can Tho University, Can Tho City, Vietnam; 3Department of Biology, College of Natural Sciences, Can Tho University, Can Tho City, Vietnam; 4College of Faculty of Food Technology - Biotechnology, Can Tho University of Technology, Can Tho City, Vietnam.
Abstract | The present study was conducted aiming to isolate and characterize bacteriophages with lytic activity against Escherichia coli infected poultry. A total of 72 samples of soil from 18 chicken farms were collected in six provinces in the Mekong Delta, Vietnam. Samples were primarily subjected to rapid detection methods, and then isolation of phage was done by a double agar layer method using E. coli as the host system. Phages were characterized on the basis of plaque morphology, pH susceptibility and host range. Results showed that the recovery of phages in soil was at a high proportion (73.6%), in which the percentage of phage isolates was higher from Noi chicken farms (66.6%) as compared to broiler chicken farms (58.3%). Four different phage morphotypes were observed against E. coli. There was a high rate of phages which existed at pH 2.0 at 26.4%. The percentage of phages that could survive from pH 5.0 to pH 3.0 significantly decreased from 84.9% to 39.6%. TEM analysis performed for MHH6 and PR2 which had widest host range, revealed that both phages belong to the Myoviridae family. It could be concluded that the MHH6 and PR2 phages have a wide host range and thus exhibit the potential to be used as a drug substitute tool against E. coli infection in chickens.
Keywords | Chicken, Escherichia coli, Infection, Isolation, Phage
Received | October 27, 2019; Accepted | January 10, 2020; Published | February 10, 2020
*Correspondence | Nguyen Trong Ngu, Department of Veterinary Medicine, College of Agriculture, Can Tho University, Can Tho City, Vietnam; Email: ntngu@ctu.edu.vn
Citation | Ngu NT, Loc HT, Nhan NTH, Huan PKN, Anh LH, Xuan NH (2020). Isolation and characterization of bacteriophages against Escherichia coli isolates from chicken farms. Adv. Anim. Vet. Sci. 8(2): 161-166.
DOI | http://dx.doi.org/10.17582/journal.aavs/2020/8.2.161.166
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2020 Ngu et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Chickens and other poultries are popular food all over the world and poultry industry has been developing rapidly in Vietnam in recent years. However, chickens and other cultivated animals are constantly threatened by diseases induced by pathogenic bacteria. Among these diseases, diarrhea in chicken caused by intestinal pathogenic Escherichia coli causes serious damages to the industry and results in vast economic losses (Roy et al., 2006). Furthermore, a large number of intestinal pathogenic E. coli or other microbes can be transmitted to other animals or even humans by some vectors, becoming significant risk factors to human health (Doyle and Erickson, 2006). To control colibacillosis in poultry, antibiotics have been widely used to fight infections. However, inappropriate or excessive use of antibiotics could lead to the emergence and spread of resistant bacterial strains, rending antibiotics treatment more and more ineffective (Sulakvelidze et al., 2001; Matsuzaki et al., 2005; Gorski et al., 2009). In Vietnam, household and small farms showed frequent antimicrobial usage associated with a high prevalence of resistance to the most commonly used antimicrobials. Given the weak biocontainment, the high prevalence of resistant E. coli could represent a risk to the environment and to humans (Nguyen et al., 2015). Therefore, considering the emergence of drug-resistant bacteria causing infectious diseases, as well as the growing concern regarding the failure of antibiotic drug discovery pipeline, introducing proper alternatives to conventional antibiotics is of paramount importance (Sulakvelidze et al., 2001).
Bacteriophages or (phages) which are bacterial viruses, the most abundant living form widely distributed in soil, deep-sea and water, may be an ideal choice for this purpose due to their ability in killing target host cell. Phages are the largest group of viruses utilizing species in the Bacteria and Archaebacteria as hosts (Rahaman et al., 2014; Kazi and Annapure, 2016). These natural viruses infect specific host bacteria and usually destroy them. Since the early days of their discovery, bacteriophages have been exploited as tools for typing bacteria, particularly those with pathogenic potential. Phage can exhibit specific patterns of host infectivity, even within isolates of a particular bacterial species. It is now recognized that phages have played a pivotal role in driving the evolution of pathogens.
In recent years, as drug-resistant strains of bacteria increased, phage therapy treating bacterial infections with bacteriophages could be a future alternative to antibiotic treatment of bacterial infections. Bacteriophage therapy has been widely attempted to treat different animal diseases induced by different types of bacteria (Sheng et al., 2006; Oliveira et al., 2010). There are, however, several problems to be solved, which are mainly associated with the biology of phages, the interaction between phages and their bacterial hosts. Therefore, the present investigation was conducted to isolate and characterize lytic bacteriophages from the soil samples in poultry farms using Escherichia coli as the host system and to assess their in vitro susceptibility in common bacterial pathogens isolated from clinical cases.
MATERIALS AND METHODS
Sample collection
Soil samples were collected at 18 poultry farms with a scale of more than 500 birds in six provinces in the Mekong Delta. In each farm, four samples were collected with approximately 10 cm deep from the top of the poultry coop ground according to the diagonal rule which was described by Sava (1994). Soil from just below the surface was placed into a tube using a sterile plastic spoon that was discarded after use. Samples were pooled in sterilized tubes at 4°C and transported to the laboratory within 24 h of collection.
Bacteria strains and growth conditions
For host range determination, the Escherichia coli isolates of O6 strain was acquired from the American Type Culture Collection (ATCC® 25922™) and two Escherichia coli strains (O1 TG1 and O78 VL1) were obtained from the collection of Thu et al. (2019) which originated from poultry illnesses. All bacterial strains were routinely cultivated at 37 oC in 24 h by using Tryptic Soy Agar (TSA).
Escherichia coli isolation
At the first stage, the homogeneous soil samples were added to a beaker with 20 ml of sterile distilled water and mix well, leaving to stand for 5 minutes. Then, 10 ml of the supernatant suspension was transferred into a sterile falcon tube. Afterwards, 20 μl of the suspension was cultured on the Tryptone Bile X-glucuronide media (TBX) for E. coli isolation (Gross and Rowe, 1985).
Isolation of bacteriophages
Soil samples for bacteriophage isolation were homogenized in 20 ml homogenization buffer (0.25 M KH2PO4 adjusted to pH 7.2 with NaOH) (Kim and Ryu, 2011) which were then were centrifuged (12000 rpm/4°C/10 min) and the supernatant was passed into an eppendorf tube containing chloroform 1.5%. Prepared soil samples were added to the cultures of previously isolated bacteria during the mid-exponential growth phase and incubated (12 h/37°C). The phage lysate was then centrifuged (6,000 rpm/4°C/5 min) and the supernatant was transferred into an eppendorf tube. Finally, the plaque assay was performed using a double-overlay method described by Kropinski et al. (2009) and a culture of earlier isolated E. coli was used for isolations. Pure isolates of bacteriophages were obtained by single plaque isolation performed in triplicate.
Spotting and overlay assay
The assays were routinely performed to form plaques and determine phage titres using TSA plates (0.5% agar for the top, 1.5% agar for the bottom) according to methods described previously (Kronpinski et al., 2009). Plaque formation was verified after incubation at 37oC for 24 h.
Host range determination
To determine the host range of all isolated phages, different bacterial strains, namely Escherichia coli of serotype O1 TG1 and O78 VL1 strains isolated from different clinical samples of poultry illnesses and O6 ATCC strain that were tested. The host range was created by observing the presence of plaques onto a double layer agar plate, prepared as described in the preceding paragraph. Ten-fold dilutions of phage stocks were performed in TSB medium and spotted on the lawn of a potential host. Plates were incubated at 37°C and examined for plaques after 18-24 h.
Plaque morphology
Plaque morphology of all coliphages was tested on E. coli O6 strain which was employed to analyse plaque morphology of Escherichia coli phages. To determine plaque size, serial ten-fold dilutions of phage stocks were prepared in TSB medium. In the next step, 1 ml of the host strain culture was mixed with 2 μl of an appropriate dilution of bacteriophage lysate and added to the 10 ml of the top TSB agar, supplemented with 0.5% agar/agarose. The mixture was poured onto a TSB plate 1.5% agar. The Petri dishes were incubated at 37°C, and after 18-24 h, plaque morphology and diameter were assessed. Morphology of plaque was recorded according to their size, edge, and boundaries (Ellis and Winters, 1969) and was noted as small (under 2 mm), medium (2 mm), and large (larger than 2 mm)/clear or diffused type plaques.
Effect of pH
The effect of an acidic pH on phage particles, therefore, was studied using TSB medium with pH from 2 to 6, according to a procedure described previously (Verma et al., 2009) in order to select bacteriophages which can be used to treat gastrointestinal diseases of chickens caused by E. coli. Phage lysate was incubated for 1 h in the given medium (at the volume proportion 1:9) at 37°C, and after the preparation of serial 10-fold dilutions, they were used for plating. To determine phage stability in various pH levels, phages incubated in the medium of pH 7.0 were used as a control. After 18 h of incubation at 37°C, the percentage of phages able to lyse the host bacterial cells was estimated.
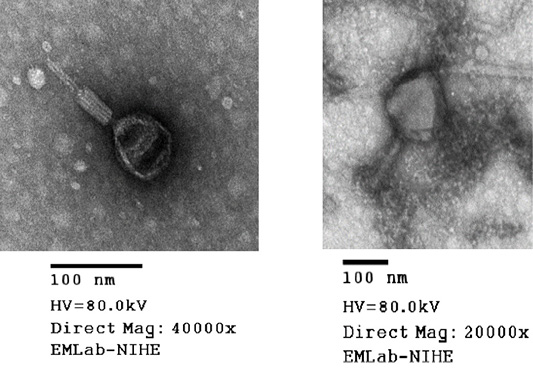
Transmission electron microscopy (TEM)
Phages were examined by transmission electron microscopy (JEOL TEM 1010, JEOL-Japan) at the National Institute of Hygiene and Epidemiology (Ha Noi, Vietnam). Based on their morphology, phages were identified and classified according to the guidelines of the International Committee on Taxonomy of Viruses (Fauquet et al., 2005).
Statistical analyses
Principal Component Analysis (PCA) was used for the analysis of morphological and biological data.
RESULTS
Escherichia coli and phages isolates
The monocultures on TBX selective media for the growth of bacteria led to the isolation of 72 strains of morphology features matching the Escherichia coli. The experiments confirmed the absolute presence of E. coli in soil in chicken farm (100%). The results also showed that substantial populations of the bacteriophages existed in the soil at the chicken farm with a high rate of 73.6% (Table 1). The presence of phages was slightly higher in soil of Noi chicken farms (66.6%) as compared to broiler chicken farms (58.3%). In the present study, a total of 53 phage isolates were obtained. For Escherichia coli host, four different phage types were assigned on the basis of plaque morphology. Plaques formed by isolated bacteriophages were shown in Figure 1. Phages that could infect and produce plaques formed small, clear plaques with the absence of halo zones, clear plaques with distinguishable halo zones, small turbid plaques.
Table 1: Status of E. coli and phages isolates in soil samples
| Source of soil sample | Number of samples |
Number of E. coli isolates (%) |
Number of phages isolates (%) |
| Noi chicken farm | 36 | 36 (100.0) | 24 (66.6) |
| Broiler farm | 36 | 36 (100.0) | 21 (58.3) |
| Total | 72 | 72 (100.0) | 53 (73.6) |
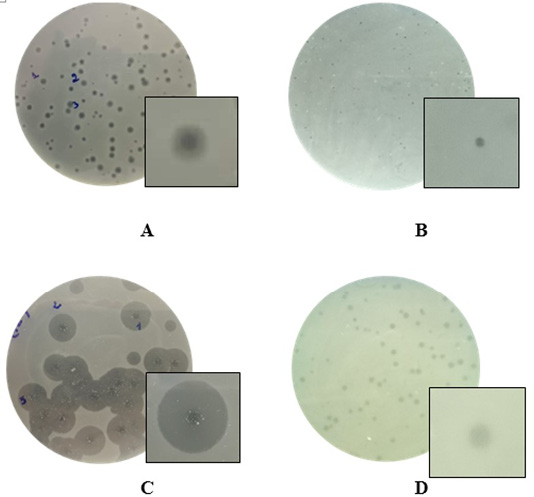
Figure 1: Examples of diversity of phage plaque morphology following morphological types of plaques are shown: plaques with a halo (A); small-sized pin-headed, clear plaque (B); plaques with clear centers and turbid edges (C), and turbid plaque (D).
Effect of pH condition on phages
The stability of phages dropped noticeably with the increase of acidic conditions. Most phages were stable in neutral pH (pH 5.0-7.0) but their numbers were reduced in acidic pH conditions after incubation. 4-fold reduction of phage numbers was recorded in pH 2.0 but some phages survived a 24 h incubation at each pH tested. The results in Table 2 showed that bacteriophages could be found in all the pH values (from 2.0 to 6.0) with differential proportions. A total of 14/53 phages (accounting for 26.4%) existed at pH 2.0, 100% of bacteriophages could exist at pH 6.0 and pH 7.0. The percentage of phages that could survive from pH 5.0 to pH 3.0 significantly decreased from 84.9% to 39.6%. Bacteriophages that could stand at pH 2.0 would be selected to the assay of lytic activity of phage lysate against E. coli.
Host range of phage isolates
The selection of lytic bacteriophages was completed based on triple passage of phage, positive in vitro multiplication, and cross-checked on the reference strain. The results of lytic activity of phage lysate against other bacteria were illustrated in Table 3. MHH6 and PR2 phages showed a relatively broad host range and yielded clear plaques on the algal lawns of E. coli O1 TG1, O78 VL1 and O6 ATCC strains while five phages (PR3, PD2, PR4, PR7, and PD2) were found effective against only two serotypes of E. coli. Among the three serotypes of E. coli tested, none of the bacteria was found sensitive to PD1. Other phages showed lytic activity against one serotype only.
Table 2: Effects of pH on the survival ability of bacteriophages.
| Survival ability of bacteriophages |
pH condition |
|||||
| 2.0 | 3.0 | 4.0 | 5.0 | 6.0 | 7.0 | |
| Number of phages | 14 | 21 | 33 | 45 | 53 | 53 |
| Proportion (%) | 26.4 | 39.6 | 62.3 | 84.9 | 100.0 | 100.0 |
Table 3: The ability of bacteriophages from the collection (phages survived at pH 2) to lyse the strains of E. coli
| No | Phage name |
E. coli strains |
Number of E. coli strains lysing |
Proportion (%) | |||
| O1 | O78 | O6 | |||||
| 1 | MHH6 | + | + | + | 3 | 100.0 | |
| 2 | PR2 | + | + | + | 3 | 100.0 | |
| 3 | PR3 | + | - | + | 2 | 66.7 | |
| 4 | PD2 | + | + | - | 2 | 66.7 | |
| 5 | PR4 | + | - | + | 2 | 66.7 | |
| 6 | PR7 | + | - | + | 2 | 66.7 | |
| 7 | PD2 | - | + | + | 2 | 66.7 | |
| 8 | MHH4 | - | - | + | 1 | 33.3 | |
| 9 | MHH3 | - | - | + | 1 | 33.3 | |
| 10 | PR1 | + | - | + | 1 | 33.3 | |
| 11 | HG9 | - | + | - | 1 | 33.3 | |
| 12 | CD3 | - | - | + | 1 | 33.3 | |
| 13 | PD5 | + | - | - | 1 | 33.3 | |
| 14 | PD1 | - | - | - | 0 | 0.0 | |
| Total | 8 | 5 | 10 | ||||
Abbreviations: (+) lysis after infection with tested bacteriophage, (-) a lack of lysis after infection with tested bacteriophage.
Transmission electron microscopy (TEM)
Virion morphology of all phages isolated from the soil samples was investigated by means of electron microscopic studies. TEM analysis performed for MHH6 and PR2 revealed that both phages belonged to the Myoviridae family based on their morphology and presence of the tail diameter value was used to classify viruses with longer tails contractile (Figure 2).
DISCUSSION
The results of this study showed that E. Coli was present in 100% of the samples. The findings were consistent with the interpretation of Winfield and Groisman (2003) and Lyautey et al. (2010) suggesting that E. coli might exist in many places such as water, farmland, and animal production systems. According to Lau and Ingham (2001), E. coli can survive for at least 19 weeks at 9-21°C in soil or cow dung. In other words, the soil was an environment for long-term storage of E. coli. E. coli could easily infect humans and animals but their presence posed an advantage to the study because it was an abundant source of hosts for the isolation of bacteriophages. For isolation of bacteriophages from soil samples, a collection of 53 phages infecting E. coli isolated strains have been created (Table 1), which were higher than the figure from manure and soil in cattle farms in Tokyo, Japan by Tanji et al. (2004). This study showed that soil samples in chicken farms have a great potential for the isolation of bacteriophages which confirms findings reported in other publications (Viazis, 2011; Grygorcewicz et al., 2015), where lytic bacteriophages were also isolated from various slurry samples. The fact that phages were isolated from the same environment as bacteria may indicate that these organisms, due to the coevolutionary dynamics between bacteria and the bacteriophage, are in balance, enabling them to co-exist in one environment (Koskella and Meaden, 2013). All phages of the study were then checked for plaque morphology type (Figure 1) because it was one of the foremost criteria for the characterization of phages (Shukla and Hirpurkar, 2011). Variation in the plaque morphology of the present study may correspond to the difference in phage strains (Tiwari et al., 2010). However, Pedroso and Martins (1995) did not find any relationship between coliphage family and specific plaque morphology.
One constraint that could limit the application of phage by the oral route is that the effectiveness of the administered phage is rapidly reduced by acid (Joerger, 2003). According to Mabelebele et al. (2014), pH in the gastrointestinal tract of chickens differed in each segment, as follows in the kites pH = 6.08 ±0.15, in the proventriculus pH = 4.65 ± 0.20, in the gizzard pH = 3.47±0.12, in the small intestine pH = 6.43 ± 0.03, in the large intestine pH = 6.40 ± 0.06 and in the caecum pH = 6.62 ± 0.16. Due to the abovementioned reason, there was a need to check the survival ability of phages in various pH values. By checking the effect of pH on the survival ability of phages isolates, the results of this study (Table 2) showed that phage isolates were sensitive to acidic media with the reduction in phage survival which might be mainly due to the denaturation of phage coat proteins caused by extreme acidity. In the research of Coffey et al. (2011), it was observed that phages e11/2 and e4/1c against E. coli reduced their viability to undetectable levels at pH 2, while phage survival was not significantly different at pH values between 3 and 10. Meanwhile, Fister et al. (2016) demonstrated that phage P100 numbers were reduced below the detection limit at pH 2.0, and after 24 h at pH 4.0 viability was not significantly reduced. Although current research witnessed a rapid decrease in the number of phages that could survive in acidic pH conditions, there still was a presence of fourteen phages at pH 2.0 which could be used for the next investigation in the lytic activity of phages against E. coli strains.
Phages were additionally investigated for their abilities to infect isolates of E. coli (O1 TG1, O78 VL1 and O6 ATCC strains). The results (Table 3) strongly suggested a possible use of these phages in further work on bacteriophage therapy, indicating that each tested E. coli strain could be infected and lysed by at least one bacteriophage from the collection (in most cases by several or many phages). In particular, MMH6 and PR2 phages which had the initial characterization of morphology (Figure 2) by TEM belong to Myoviridae families could be used for bacteriophage therapy treatment in E. coli infection in chickens. Wide host ranges reported in the present study were in conformity to the reports of Bielke et al. (2007) who observed that the phage host range is not always genera restricted, so phages could have wide host range. The present observations were in partial conformity with Carey-Smith et al. (2006) who had reported narrow range phages restricted to a maximum of two bacterial species. During the last decade, a marked increase in the number of identified phages has been observed when phages were recorded against E. coli and other enterobacteria (Marwa and Abdulamir, 2014).
Conclusions
In conclusion, this study isolated and characterized phages at the high rate from soil samples in chicken farms by using E. coli as the host system. The features of the investigated phages, including the ability of survival in various pH values, particularly MHH6 and PR2 phages, give them the potential to be used in in phage therapy for treatment E. coli infection in chickens.
Acknowledgments
This research is funded by the Ministry of Education and Training, Vietnam under grant number B2018-TCT-32.
Authors’ Contributions
NTN and PKNH contributed to the design of the study; NTHN, LHA and NHX conducted the data collection and analysis. NTN and HTL prepared the manuscript. All authors have read and approved the final manuscript.
Conflict of interest
The author declare there is no conflict of interest.
References