Advances in Animal and Veterinary Sciences
Research Article
Clostridium difficile Infections in Adult Horses and Foals: Prevalence and Associated Risk Factors
Abd El Karem Mansour Morsi1, Ibrahim Elsohaby1,2*, Manar Abdelmageed3, Theeb Al- Marri4, Mahmoud Fayez4,5
1Department of Animal Medicine, Faculty of Veterinary Medicine, Zagazig University, Zagazig City, Sharkia Province, Egypt; 2Department of Health Management, Atlantic Veterinary College, University of Prince Edward Island, Charlottetown, Prince Edward Island, Canada; 3Department of Pathology, Faculty of Veterinary Medicine, Zagazig University, Zagazig City, Sharkia Province, Egypt; 4Ministry of Agriculture, Al Ahsa Veterinary Diagnostic Laboratory, Saudi Arabia; 5Veterinary Serum and Vaccine Research institute, Ministry of Agriculture, Cairo, Egypt.
Abstract | Clostridium difficile (C. difficile) is one of the main causes of diarrhea and enterocolitis in horses. The goal of this study was to investigate the prevalence and risk factors associated with C. difficile infections in adult horses and foals with and without diarrhoea. Fresh faecal samples were collected from 407 horses originating from 35 stables in Eastern province, Saudi Arabia. The collected samples were cultured to isolate C. difficile. ELISA was used for detection of C. difficile toxins (A and B). Intestinal and cecal samples were collected from two dead horses for histopathological examination. C. difficile were detected in a total of 24 (5.9%) horses and its toxins were detected in 13 (54%) isolates. Toxigenic C. difficile infections (positive cases) were closely associated with diarrhetic foals than normal adult horses. Furthermore, there was close association between toxigenic C. difficile infections and signs of colic and bloody faeces. The frequency of positive cases was higher in horses under antibiotic therapy than those without treatment. The study concludes that acute colitis in adult horses and diarrhea in foals previously treated with antibiotics is associated C. difficile infection.
Keywords | Clostridium difficile, Horses, Foals, Diarrhea, Risk factors
Received | September 19, 2019; Accepted | October 26, 2019; Published | December 12, 2019
*Correspondence | Ibrahim Elsohaby, Department of Health Management, Atlantic Veterinary College, University of Prince Edward Island, Charlottetown, Prince Edward Island, Canada C1A 4P3; Email: ielsohaby@upei.ca
Citation | Morsi AEKM, Elsohaby I, Abdelmageed M, Al-Marri T, Fayez M (2019). Clostridium difficile infections in adult horses and foals: Prevalence and associated risk factors. Adv. Anim. Vet. Sci. 7(s2): 169-174.
DOI | http://dx.doi.org/10.17582/journal.aavs/2019/7.s2.169.174
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Morsi et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Enterocolitis is a severe sporadic disease associated with high morbidity and mortality rates in foals and adult horses (Bäverud et al., 1997). The etiology is often unclear however, Clostridium difficile (C. difficile) has frequently been associated with colitis in adult horses (Songer et al., 2009) and diarrhea in foals, which can occur spontaneously or may be associated with antimicrobial use (Båverud, 2004; Silva et al., 2012). C. difficile is a spore-forming Gram-positive anaerobic bacterium, which produces two major toxins: A (enterotoxin = TcdA) and B (cytotoxin = TcdB) (Vedantam et al., 2012). Adult horses and foals are equally susceptible to C. difficile infection (Jones et al., 1988; Madewell et al., 1995; Weese et al., 1999). Clinical signs and lesions of C. difficile-associated disease (CDAD) may be characteristic but are not pathognomonic. The distribution of the intestinal tract lesions is dependent on the animal age (Perrin et al., 1993; Uzal et al., 2012). In foals, C. difficile infection lesions are mostly limited to the small intestine; while in adult horses, the lesions are usually limited to the cecum and colon (Perrin et al., 1993).
Clinical history and signs can be used for presumptive diagnosis of equine CDAD (Diab et al., 2013). The CDAD should be considered in any horse with a history of antibiotic treatment and showed signs of diarrhea, enteritis or enterocolitis (Gustafsson et al., 2004; Keel and Songer, 2006). Some researchers considered the isolation of C. difficile from faeces and/or intestinal contents is a diagnostic for CDAD in horses (Jones et al., 1988; Wolfhagen et al., 1994). However, other researchers revealed that isolation alone is not confirmatory due to the circulation of non-toxigenic strains of C. difficile (Lyerly et al., 1998). Identification of toxigenic strains of C. difficile can be performed via detection of toxins using ELISA (Weese et al., 2001) or detection of toxin genes by PCR (Kato et al., 1991; Wolfhagen et al., 1994; Groschel, 1996). The ELISA is more commonly used for diagnosis of CDAD because of its high sensitivity and specificity, low cost and technical ease (Whittier et al., 1993; Lyerly et al., 1998).
The data available about CDAD in horses in Saudi Arabia is scarce. Most studies investigated the risk factors associated with the development of CDAD in equine were focused on two major risk factors; hospitalization and antibiotic treatment (Baverud, 2004; Baverud et al., 2003; Madewell et al., 1995). These studies excluded the risk factors related to the horse health status and stable related factors. Therefore, the aim of the present study was to explore the prevalence and associated risk factors with C. difficile infections in adult horses and foals with and without diarrhoea in Saudi Arabia.
Materials and Methods
Sampling and data collection
A total of 407 faecal samples were collected from horses originating from 35 stables in Eastern province, Saudi Arabia. All horses involved in the study were Arabian horses and were used in local horse races and shows. The age of horses at sampling ranged from 6 days to 60 months. The examined animals were 91 foals (≤ 6 months) and 316 adult horses (> 6 months). Based on the clinical signs, horses were divided into diarrheic (n = 49) and normal (n = 42) foals, and diarrheic (n = 30) and normal (n = 286) adult horses. Two hundred thirty-six horses were treated with antibiotics including 15 horses were treated with ampicillin (20 mg/Kg for 5 days); 142 horses were treated with cephalosporin (2.2 mg/Kg for 3 days); 20 horses were treated with gentamicin (6.6 mg/Kg for 5 days), and 59 horses were treated with tetracycline (10 mg/Kg for 5 days). Faecal samples were aseptically collected from rectum and placed in thick plastic bags and transmitted to the laboratory within few hours. Additionally, two horses had a history of colic and diarrhea were dead. Intestinal contents, intestinal and cecal specimens from dead horses were collected during the post-mortem examination. Faecal samples and intestinal contents were divided into two portions: 5 g for C. difficile culture and 10 g stored at -70 °C for further detection of C. difficile toxins. However, the intestinal and cecal specimens were kept in 10% formalin for histopathological examination.
The sample collection was supplemented with an epidemiological questionnaire to gather data on horses including age, gender, observations of clinical signs (i.e. diarrhoea, colic and bloody faeces), antibiotic treatment, type of antibiotics used in treatment, stable ground type and the use of disinfectants. The study protocol was approved by the Ethics Committee according to local and national regulations. All respondents gave an oral/written consent to participate in the study.
Isolation of C. difficile
Two grams of each sample (faeces or intestinal contents) were inoculated into C. difficile moxalactam and norfloxacin broth (CDMN) (Oxoid, UK) and incubated at 37 °C for 5 to 7 days under anaerobic condition. An aliquot of the broth was alcohol shocked with anhydrous ethanol for one hour. Pellets were collected by centrifugation at 3800 g for 10 min and streaked into a C. difficile monolactam norfloxacin (CDMN) agar plate, then incubated anaerobically for 48 h at 37 °C. The C. difficile suspected colonies were sub-cultured into 5% sheep blood agar and incubated as above for purification. Purified colonies were initially identified by Gram staining, and then the production of proline aminopeptidase was determined using PRO disc (Remel, USA) following the previously published protocol (Fedorko and Williams, 1997). Colonies which showed Gram-positive bacilli and hemolysis on blood agar were biochemically identified by automated VITEK-2 Compact system (BioMerieux, Marcyl’Etoile, France) using VITEK-2 ANC ID card.
Detection of C. difficile toxins
Confirmed C. difficile isolates were inoculated in TY medium (3% w/v tryptose, 2% w/v yeast extract, 0.1% w/v thioglycollate, pH 7.4) and incubated anaerobically at 37 °C for 48 h. C. difficile toxins A and B were determined first in the culture supernatants and also in the faecal samples and intestinal contents using a commercial ELISA kit (RIDASCREEN® Clostridium difficile Toxin A/B, R-Biopharm AG, Germany) according to following the manufacturer’s instructions.
Data analysis
The prevalence of C. difficile and C. difficile toxins in diarrheic and normal adult horses and foals were estimated from the proportion of positive to total number of examined samples. Due to the small number of cases where C. difficile toxins were detected, Fisher’s exact test was used to assess the unconditional associations between cases (toxin positive) and independent variables (age, gender, signs of diarrhea, signs of colic, antibiotic treatment, isolation of infected horses, stable ground types and use of disinfectant). In addition, correspondence analysis was used to evaluate the association between the outcome and categorized independent variables (Greenacre, 2017). All data were analyzed using STATA version 15 for Windows (Stata Corp., College Station, TX, USA).
Results
Clinical and post-mortem examination
The most common signs detected during physical examination were fever, diarrhea, bloody faeces, colic, tachycardia, tachypnea and dehydration. At necropsy, the gross pathological findings were diffuse darker discoloration of segments of the colon together with petechial hemorrhages and thickening of the wall of the colon with edema (Figure 1). The mucosal surface was congested and hemorrhagic. The contents of the large intestine were watery, brown and foul-smelling. Mesenteric lymph nodes were moderately enlarged, edematous and hemorrhagic. Histopathological findings (Figure 2) were mainly coagulative necrosis of the mucosa and marked mucosal and submucosal thrombosis. Hemorrhages, fibrinous exudate and leukocytic infiltration were seen throughout the mucosa and submucosa. Focal mucosal ulcerations and fibrinonecrotic pseudomembranes were also observed.
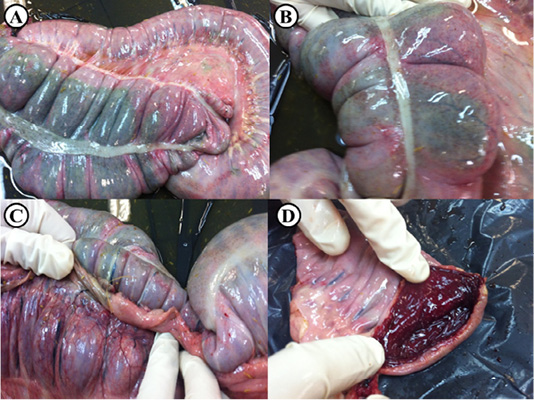
Figure 1: Post-mortem lesions of an adult horse suspected to be infected with C. difficile. (A) Diffuse darker discoloration of the serosal surface of the colon, (B) Sub-serosal petechiae are seen on the affected colon, (C) Colonic wall blood vessels are severely congested, (D) Opened colon shows marked congestion and hemorrhage of the mucosal surface.
C. difficile isolation and toxins detection
Clostridium difficile were detected in a total of 24 (5.9%) examined horses and only 13 (54%) of the isolates were toxin producers. C. difficile was isolated from 7 (23.3%) of diarrheic adult horses and 3 (1.05%) of normal adult horses. However, only 4 (57%) C. difficile isolated from diarrheic adult horses were toxin producers (Table 1). C. difficile was isolated from the faeces of 11 (22.5%) foals with diarrhea and from only 3 (7.1%) apparently healthy foals. Nine (82%) of C. difficile isolates were detected in faecal samples of diarrheic foals were toxigenic. However, isolates from normal foals did not produce any toxins.

Figure 2: Histopathological findings in an adult horse suspected to be infected with C. difficile. (A) Colon showing coagulative necrosis of the mucosa and prominent mucosal and submucosal thrombosis (inset), (B) Colon showing congestion and necrosis in addition to diffuse infiltration of leucocytes and fibrinous exudate (inset).
Table 1: Detection of C. difficile and C. difficile toxins in diarrheic and normal adult horses and foals.
| Animals | No. | No. of C. difficile isolates (%) | No. of C. difficile toxins (%) | |
| Adult horses | ||||
| Diarrheic | 30 | 7 (23.3%) | 4 (57%) | |
| Normal | 286 | 3 (1.1%) | 0 (0%) | |
| Total | 316 | 10 (3.2%) | 4 (40%) | |
| Foals | ||||
| Diarrheic | 49 | 11 (22.5%) | 9 (82%) | |
| Normal | 42 | 3 (7.1%) | 0 (0%) | |
| Total | 91 | 14 (15.4%) | 9 (64%) | |
| Total | 407 | 24 (5.9%) | 13 (54%) | |
Risk factors
A total of 407 horses with a higher proportion of adult (77.6%) than foals (22.4%) were included in this study (Table 2). Approximately, 19.4% of horses had diarrhea; 3.4% with bloody faeces and only 4.7% showed signs of colic. Examination of fecal samples revealed that 13 out of 407 (3.2%) of the horses were positive for toxigenic C. difficile. The association between toxigenic C. difficile infections and prospective risk factors was tested by Fisher’s exact test and presented in Table 2.
Figure 3 shows the risk factors associated with the toxigenic C. difficile cases among horses. Toxigenic C. difficile infections (positive cases) were strongly linked with diarrheic foals than normal adult horses. The graph also shows the close association between toxigenic C. difficile infections and signs of colic and bloody faeces. On the other hand, the absence of C. difficile infections was associated with normal adult horse, not isolated and lived in concert disinfected stables.
Table 2: Prevalence of C. difficile toxins in diarrheic and normal adult horses and foals.
| Factors | No. examined |
No. positives |
Prevalence (%) |
95% CI* |
P- value * |
| Age | |||||
| Foals | 91 | 9 | 9.9 | 4.6-17.9 | 0.0001 |
| Adult | 316 | 4 | 1.3 | 0.3-3.2 | |
| Gender | |||||
| Male | 125 | 7 | 5.6 | 2.3-11.2 | 0.077 |
| Female | 282 | 6 | 2.1 | 0.8-4.6 | |
| Horses with diarrhea | |||||
| Yes | 79 | 13 | 16.5 | 9.1-26.5 | 0.0001 |
| No | 328 | 0 | 0 | - | |
| Horses with colic | |||||
| Yes | 19 | 10 | 52.6 | 28.9-75.6 | 0.0001 |
| No | 388 | 3 | 0.8 | 0.2-2.2 | |
| Horses with bloody faeces | |||||
| Yes | 14 | 10 | 71.4 | 41.9-91.6 | 0.0001 |
| No | 393 | 3 | 0.8 | 0.2-2.2 | |
| Horses with antibiotic treatment | |||||
| Yes | 236 | 12 | 5.1 | 2.7-8.7 | 0.035 |
| No | 171 | 1 | 0.6 | 0.01-3.2 | |
| Antibiotic used | |||||
| No | 171 | 1 | 0.6 | 0.01-3.2 | 0.05 |
| Ampicillin | 15 | 0 | 0 | - | |
| Cephalosporin | 142 | 11 | 7.7 | 3.9-13.4 | |
| Gentamicin | 20 | 0 | 0 | - | |
| Tetracycline | 59 | 1 | 1.7 | 0.04-9.1 | |
| Isolation of infected horses | |||||
| Yes | 183 | 10 | 5.5 | 2.7-9.8 | 0.03 |
| No | 224 | 3 | 1.3 | 0.3-3.9 | |
| Stable ground type | |||||
| Sand | 224 | 12 | 5.4 | 2.8-9.2 | 0.026 |
| Concrete | 183 | 1 | 0.6 | 0.01-3.0 | |
| Use of disinfectants | |||||
| Yes | 102 | 7 | 6.9 | 2.8-13.6 | 0.022 |
| No | 305 | 6 | 2.0 | 0.7-4.2 | |
| Total | 407 | 13 | 3.2 | 1.7-5.4 | |
* CI: confidence interval; P-value resulting from Fisher’s Exact test considered significant at 0.05
Discussion
This study investigated the prevalence of C. difficile infections and the associated risk factors in diarrheic and normal adult horses and foals. The total prevalence of C. difficile reported in the present study was higher (5.9%) than reported previously (4.6%) for similar population (Silva et al., 2012); however much lower than again previously reported (23%) from diarrheic horses in Australia (Thean et al., 2011). The variations in the prevalence rates of C. difficile between studies are attributed to the differences in design of each study, sensitivity and specificity of diagnostic tests, sample collection, predisposing factors, animal age and regional or temporal prevalence (Baverud et al., 2003; Wesse et al., 2001; Diab et al., 2013).
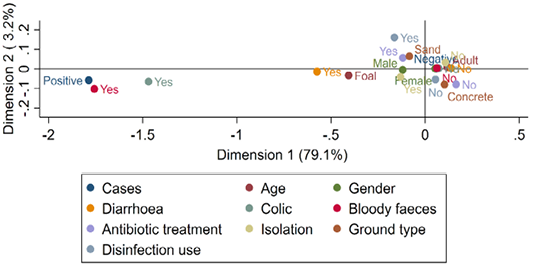
Figure 3: Multiple correspondence analysis of risk factors associated with the presence of C. difficile toxins in diarrheic and normal adult horses and foals.
From the adult horses with diarrhea, 7 (23.3%) of faecal samples were culture-positive for C. difficile and only 4 (57%) isolates were positive for toxin-production. This result is consistent with the results of a previous study (Baverud et al., 2003) where C. difficile was isolated from 5 (100%) adult horses with signs of colic however only 3 (60%) isolates were positive for C. difficile toxins production. Previous studies have reported link between C. difficile infection and acute colitis in adult horses (Cosmetatos et al., 1994; Madewell et al., 1995; Gustafsson et al., 1997; Båverud et al., 1998). Fecal samples from 3 normal adult horses were C. difficile culture-positive, but these isolates were non-toxigenic. These horses did not show any signs of enteric disorders and confirm that CDAD is associated with toxin-production (Lyerly et al., 1998; Diab et al., 2013).
An interesting finding of this study was that the age of C. difficile culture-positive foals ranged from 6 days to 2 months, whereas foals aged from 2 to 6 months were culture-negative. Similar result was previously revealed by Baverud et al. (2003) who reported C. difficile infections were recorded in foals less than one month old. In recent years, C. difficile has been associated with diarrhea in foals (Jones et al., 1988; Magdesian et al., 1999; Weese et al., 2001). In the present study, C. difficile has been isolated from 11 (22.5%) foals with diarrhea and only 9 (82%) isolates were toxins producers. However, only 3 (7.1%) normal foals were carriers for C. difficile.
The present study showed that CDAD cases were mainly associated with diarrheic foals. This finding is in line with previous researches reporting a high risk of C. difficile infection in foals (Silva et al., 2012; Weese et al., 2001). Although, the number of CDAD cases is low (13), the frequency of C. difficile positive cases between horses have signs of colic and bloody faeces was higher than normal horses as was previously reported (Baverud et al., 1997). Moreover, CDAD cases were associated with antibiotic use. The frequency of positive cases was higher in horses under antibiotic treatment than that without treatment. Similarly, several studies have reported the strong association between antibiotic use and C. difficile infection (Diab et al., 2013; Arroyo et al., 2004; Divers, 2002). This might be attributed to the adverse effects of antibiotics on number and distribution of colon and cecum normal bacteria and protozoa, which can permit the C. difficile overgrowth (Baverud, 2004; Papich, 2003; Vollaard and Clasener, 1994). The use of cephalosporins in treatment of horses involved in the present study was associated with the risk of C. difficile infection. However, other studies have reported that the most frequent antibiotics associated with C. difficile infection in horses were erythromycin, trimethoprim/sulfonamides, rifampicin, clindamycin, and gentamicin (Arroyo et al., 2004; Baverud et al., 2003; Divers, 2002; Gustafsson et al., 1997; Magdesian et al., 2002).
Conclusions
Clostridium difficile is associated with acute colitis in adult horses, diarrhea in foals and those who are previously treated with antibiotics. Isolation of C. difficile from normal adult horses and foals highlights the importance of carriers in dissemination of infection.
Acknowledgements
The authors thank horse owners who agreed to use their horse’s samples in this study. Authors also would like to thank the staff of the Al Ahsa Veterinary Diagnostic Laboratory, Saudi Arabia for technical assistance and samples collection.
Authors Contribution
All authors designed the study. Theeb Al- Marri, Mahmoud Fayez collected the samples and carried out the laboratory practical work. Ibrahim Elsohaby, Abd El Karem Mansour Morsi and Manar Abdelmageed analyzed the data and drafted the manuscript. The final manuscript was read and approved by all authors.
Conflict of interest
The authors declare that there is no conflict of interests to disclose regarding the publication of this paper.
References





