Advances in Animal and Veterinary Sciences
Research Article
Prevalence of Different Mould Genera in Meat and Meat Products with Some Reduction Trials using Essential Oils
Amina H.A. Habashy1*, Wageh S. Darwish2, Mohamed A. Hussein2, Waiel M.Salah El-Dien1
1Food Control Department, Animal Health Research Institute, Zagazig Branch, Egypt; 2Food Control Department, Faculty of Veterinary Medicine, Zagazig University, Egypt.
Abstract | This study aimed to observe the hygienic status of raw meat, fresh minced meat, luncheon, beef burger and sausage (40 each) retailed in Zagazig city, Sharkia Governorate, Egypt. Total mould count, prevalence of mould species as well as the ability of the most fungal isolates to produce lipase and protease enzymes had been conducted. The antifungal potential of clove, thyme, garlic (0.5%, 1%) essential oils and potassium sorbate 0.3% had been evaluated. The highest mean (log10 cfu/g) total mould count (2.85 ± 0.12) was recorded in sausage. The most prevalent species recovered from the examined meat and meat product samples were Aspergillus, Penicillium and Cladosporium while Alternaria, Fusarium, Mucor, Rhizopus, Sporotricum, Thamnidium and Curvularia species were recovered at low percentages. A. niger was the most predominant Aspergillus spp isolated from sausage, luncheon, raw meat, fresh minced meat and beef burger with prevalences of 50%, 45.5%, 44.4%, 41.4% and 33.3% respectively. Among 262 tested isolates, 216 (82.4%) could produce protease, 233 (88.9%) produce lipase. Essential oils caused significant inhibition on fungal growth. Clove (Syzygium Aromaticum) oils 1% and thyme (Thymus Vulgaris) oils 1% were found to be more effective than the others, although Clove 1% gave the best result in inhibition of A. flavus growth, but the odour of thyme is more palatable than that of clove. In conclusion, the obtained results revealed inadequate hygienic measures during transportation, processing, storage and distribution of meat and meat products. Therfore, strict hygienic practices should be followed. Moreover, clove, thyme and garlic (Allium Sativum) have the potential to be used as flavoring and natural preservatives in food.
Keywords | Mould, Essential oils, Antifungal effect, Meat products, Lipase, Protease
Received | September 19, 2019; Accepted | October 26, 2019; Published | December 12, 2019
*Correspondence | Amina H.A. Habashy, Food Control Department, Animal Health Research Institute, Zagazig Branch, Egypt; Email: katkooot_moftaris2008@yahoo.com
Citation | Habashy AHA, Darwish WS, Hussein MA, El-Dien WMS (2019). Prevalence of different mould genera in meat and meat products with some reduction trials using essential oils. Adv. Anim. Vet. Sci. 7(s2): 79-85.
DOI | http://dx.doi.org/10.17582/journal.aavs/2019/7.s2.79.85
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Habashy et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Meat is an essential food because it is tasty, easy to be digested and contain high percentage of good quality protein, relatively high lipid content for energy. The muscle tissue in general is an excellent source of vitamins B complex. Meat products are considered as rich source of animal derived protein, essential and none essential fatty acids, vitamins and minerals. It considered a favorite food as it’s easy to buy, fast to cook so it’s the first choice for many people to eat. Additionally unique aroma and flavour of meat make them highly attractive, especially for children (Al-Dughaym, 2010). Mould contamination of meat products indicated improper sanitary and hygienic conditions during handling, processing and storage. Adding of bad or inferior quality of flavoring agents may increase the load of contamination of such products with mould. Flavourings, such as spices, added in formulation of meat products can considerably increase the mould contamination of the meat products (Gourama and Bullerman, 1995). Enzymatic activities are natural processes in the meat after rigor mortis and increase due to temperature abuse (Tauro et al., 1986). Essential oils (EOs) generally recognized as safe (GRAS) produced by the secondary metabolism of herbal plants and spices had been used in human consumption as food additives for flavorings, antioxidant and antimicrobial. Moreover, it inhibits lipid oxidation in food of animal origin (Zengin and Baysal, 2014). Clove is one of the most valuable spices that were used for centuries as a food preservative and for many medicinal purposes (Cortés-Rojas et al., 2014). Thyme is widely consumed worldwide as a flavouring agent in foods. Recently, thyme oil possesses sufficient antimicrobial and antioxidant activity in some foods to extend the shelf-life of meat products (Assiri et al., 2016). It is known that plant extracts reduce fungal growth, as it damage cell membrane and inhibit the synthesis of protein, lipids and nucleic acids (Bayan et al., 2014). Potassium sorbate is one of the most effective food preservatives in controlling mould growth and it was used as preservative in numerous processed food products owing to its system of double bonds (Ferrand et al., 2000). The sensory characteristics considered as a main topic judging purchasing decision of the food consumer (Calkins and Hodgen, 2007).
Hence, present study throws a light on the prevalence of mould in raw meat, fresh minced meat, luncheon, beef burger and sausage retailed for sale in many districts. The ability of the isolated mould genera to produce lipase and protease enzymes was also detected. In addition, the antifungal effect of clove, thyme, garlic, natural EOs and potassium sorbate in the contaminated minced meat and the enhancement of shelf life of these meats was evaluated.
Materials and methods
Samples collection
Two hundred samples of raw meat, fresh minced meat, luncheon, beef burger and sausage (40 each) under different trade names were randomly collected from different supermarkets and butchers in Zagazig city, Sharkia Governorate, Egypt. Samples were kept in sterile polyethylene bags and preserved in an ice box then transferred to the laboratory under complete aseptic condition without delay to be examined mycologicaly as quickly as possible.
Preparation of samples
Twenty five grams of each sample were aseptically homogenized in 225 ml of 0.1% sterile buffered peptone water at 2500 rpm for 2 min using a sterile homogenizer (APHA, 2001). Such, homogenate represents the dilution of 10-1, and then decimal dilutions were done.
Determination of the total mould count
The total mould count was determined by culturing duplicate plates on each of malt extract agar media (MEA) (Oxoid), and incubated at 25 °C for five to seven days (APHA, 2001). During the incubation time, the plates were examined evrey day for the star-shape mold growth. Mould colonies were picked up under aseptic conditions and subsequently subcultured on MEA slopes and kept for further examination.
Identification of the isolated moulds
The identification of the mould colonies was carried out by careful observation and measurements of the macroscopic and microscopic characteristics of colonies which were recorded on data sheets (Pitt and Hocking, 2009).
Macroscopical examination
Observations were made for the consistency of the surface growth; the pattern of folding (rugae); the distinctness of the colony margin and for the presence of pigment either on the surface or the reverse of the colony or diffusing into the surrounding medium. Both the surface and back side of the colony were examined using a magnifying hand lens.
Microscopical examination
Briefly, using mycological needles, the part of colony was distributed with few drops of lactophenol cotton blue stain, then covered with a clean cover slide.The slides were examined using low power and high power magnification lenses for examination of micromorphological characters,concerning the head, vesicle, sterigmata, conidiophore and conidia.
Evaluation of lipolytic and proteolytic activity of isolated moulds
Lipolytic activity
The mould lipolytic activity for Tween 80 was measured according to previously described method (Ullman and Blasins, 1974). Presence of opaque zone surrounding the colony due to calcium salt crystals formation of the oleic acid liberated by the mould enzyme within 7 days at 30 °C.
Proteolytic activity
Proteolytic activity was tested on casein hydrolysis medium as described by (Paterson and Bridge, 1994). This medium contains skim milk that gives an opaque final medium. Hydrolysis of the casein, which may also be due to acid production, results in a clear zone surrounding the fungal colony at 30 °C for 7 days.
Sensory evaluation
It was carried out according to (Pearson and Tauber, 1984). A 9 point hedonic scale (9= Excellent, 8= Very very good, 7= Very good, 6= Good, 5=Medium, 4=Fair, 3=Poor, 2=Very poor, 1=Very very poor) was used for the evaluation of the overall acceptability.
Evaluation of clove, thyme and garlic EOs and potassium sorbate as antifungals in minced meat
The EOs of clove, thyme and garlic were obtained from National Research Center, Dokki, Giza. Potassium sorbate was obtained from Al Gomhoria Company at Zagazig, Sharkia Governorate, Egypt.
Preparation of minced meat
One thousand grams (1000g) fresh minced meat was purchased from the butcher’s shop, transferred to the laboratory in an ice box. Then, divided into 8 subgroups of equal amounts (125 g each) 1st subgroup as control, 2nd ,3rd,4th,5th,6thand7th subgroups were further mixed with an appropriate volume of clove, thyme, garlic EOs concentrations (0.5 and1 % v/w), respectively and 8th subgroup was further mixed with an appropriate volume of 0.3% potassium sorbate. All samples were stored at 4 °C. Sampling was carried out every 3 days until the end of the storage (9 days).
Statistical analysis
Mould counts were transferred into base-10 logarithms of CFU/g. Data was analyzed using one-way ANOVA procedure of SPSS v.23 (SPSS Inc., Chicago, Illinois, USA), after verifying normality using Shapiro-Wilk’s test. The Tukey’s multiple comparison tests was used to test for significant differences between mean values. Variation in the data was expressed as means ±SE, and the alpha level for determination of significance was set at 0.05.
Table 1: Mean values of total mould count (log 10 CFU/g) of the examined meat and meat product samples.
| Samples (n= 40 per each) | Positive samples | Min. | Max. | Mean ±S.E | |
| No. | % | ||||
| Meat | 18 | 45 | 1.00 | 3.30 |
2.11± .15c |
| Minced meat | 20 | 50 | 1.00 | 4.04 |
2.21± .20c |
| Luncheon | 25 | 62.5 | 1.00 | 3.70 |
2.41 ± .14bc |
| Burger | 32 | 80 | 2.00 | 3.70 |
2.69 ± .09ab |
| Sausage | 33 | 82.5 | 2.00 | 4.30 |
2.85 ± .12a |
a,b,c Different superscripts within each column indicate significant differences (P<0.05).
RESULTS
The results illustrated in Table 1 shows that the highest mould count was recorded in sausage samples (2.85± 0.12 CFU/g) and the lowest count was obtained in raw meat samples (2.11±0.15 CFU/g). The data obtained from (Table 2) declared number and percentage of mould genera founded that 10 mould genera could be isolated and identified from the examined samples. The identified mould genera were; Aspergillus, Penicillium, cladosprium, sporotricum, Alternaria, Fusarium, Rhizopus, Mucor, Thamnidium, and Curvularia. The most predominant mould genera in examining samples were; Aspergillus, Penicillium and Cladosporium. However, Aspergillus species were the most prevalent species in beef burger, luncheon, fresh minced meat, raw meat and sausage samples in 49%, 47.8% 46%, 42.9%, and 41.7% respectively. Penicillium species were recovered in 25.5%, 25%, 23.9%, 20.6% and 19% from burger, sausage, luncheon, minced meat and meat samples, respectively. In addition, 6 Aspergillus species were identified from meat and meat product samples. The predominant isolated species of Aspergillus were A. niger 50%, 45.5%, 44.4%, 41.4% and 33.3% from sausages, luncheon, meat, minced meat and beef burger respectively followed by A. flavus 34.5%, 31.8%, 29.6%, 26.7% and 22.2% in minced meat, luncheon, beef burger, sausage and meat respectively. Only one isolate of A. fumigatus could be isolated from luncheon, A. parasiticus from sausage, luncheon, A.ochraceus from minced meat, meat and A. terreus from luncheon, beef burger, sausage (Table 3). The ability of mould isolates for protease and lipase enzymes production was assessed.The results declared that protease and lipase enzymes were detected among 262 of them, 216 (82.4%) could produce protease, 233 (88.9%) produce lipase as shown in Table 4. Concerning organoleptic examination the experiment revealed that colour, odour and texture of the control sample changed at 3rd day. The sensory properties of treated minced beef samples chilled at 4ºC were highly acceptable under different treatments (Table 5). High scores from the panel of judges given to thyme1% treated minced beef samples while odour of clove 1% and garlic1% take fewer scores than thyme 1%. Although a panel of judges still gave high scores till 6th day and 9th day, but mean values of total A. flavus count in clove 0.5%, thyme 0.5%, garlic 1%, garlic 0.5% and potassium sorbate 0.3% increased gradually but still lesser than control at 0 times. The results illustrated in Table 6 showed that samples treated by clove 1% and thyme 1% showed a decrease in mean values of total A. flavus through 3rd day and 6th day until 9th day not detected. Clove 0.5%, thyme 0.5%, garlic 1%, garlic 0.5% and potassium sorbate 0.3% showed decreases in mean values of total A. flavus through the 3rd day in which the lowest count recorded, then started to increase gradually but still lower than a count of control in 0 day which explained that all treated sample enhanced than the control which have a higher count in 3rd day and give bad scores in organoleptics and decomposed in 6th day.
Discussion
Mould contamination of meat and meat products may occur during slaughtering of the animals, transportation, or during processing of meat products by the use of contaminated equipments or contaminated additives and spices which considered the most important source of mould contamination in meat products (Jay et al., 2005).
In the present study, as shown in Table 1 the results of the total mold count of luncheon samples nearly similar to
Table 2: Number and percentage of the identified mould genera from examined meat and meat product samples.
| Mould genera | Sausage | Beef burger | Luncheon | Minced meat | Raw meat | Total | ||||||
| No | % | No | % | No | % | No | % | No | % | No | % | |
| Aspergillus | 30 | 41.7 | 27 | 49 | 22 | 47.8 | 29 | 46 | 18 | 42.9 | 126 | 45.3 |
| Penicillium | 18 | 25 | 14 | 25.5 | 11 | 23.9 | 13 | 20.6 | 8 | 19 | 64 | 23 |
| Cladosporium | 7 | 9.7 | 4 | 7.3 | 3 | 6.5 | 4 | 6.3 | 5 | 11.9 | 23 | 8.3 |
| Sporotricum | 3 | 4.2 | 3 | 5.5 | 2 | 4.3 | 4 | 6.3 | 0 | 0 | 12 | 4.3 |
| Alternania | 6 | 8.3 | 0 | 0 | 0 | 0 | 3 | 4.8 | 3 | 7.1 | 12 | 4.3 |
| Fusarium | 2 | 2.8 | 1 | 1.8 | 1 | 2.2 | 3 | 4.8 | 2 | 4.8 | 9 | 3.2 |
| Rhizopus | 4 | 5.7 | 0 | 0 | 0 | 0 | 3 | 4.8 | 2 | 4.8 | 9 | 3.2 |
| Mucor | 0 | 0 | 3 | 5.5 | 3 | 6.5 | 0 | 0 | 1 | 2.4 | 7 | 2.5 |
| Thamnidium | 2 | 2.8 | 3 | 5.5 | 4 | 8.7 | 3 | 4.8 | 2 | 4.8 | 14 | 5 |
| Curvularia | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1.6 | 1 | 2.4 | 2 | 0.72 |
| Total | 72 | 100 | 55 | 100 | 46 | 100 | 63 | 100 | 42 | 100 | 278 | 100 |
Table 3: Incidence of Aspergillus species in examined meat and meat product samples.
|
Aspergillus species |
Sausage | Beef burger | luncheon | Mincedmeat | Meat | Total | ||||||
| No | % | No | % | No | % | No | % | No | % | No | % | |
| A. flavus | 8 | 26.7 | 8 | 29.6 | 7 | 31.8 | 10 | 34.5 | 4 | 22.2 | 37 | 29.4 |
| A. niger | 15 | 50 | 9 | 33.3 | 10 | 45.5 | 12 | 41.4 | 8 | 44.4 | 54 | 42.9 |
| A.fumigatus | 3 | 10 | 3 | 11.1 | 1 | 4.5 | 2 | 6.9 | 2 | 11.1 | 11 | 8.7 |
| A.parasiticus | 1 | 3.3 | 4 | 14.8 | 1 | 4.5 | 2 | 6.9 | 1 | 5.6 | 9 | 7.1 |
| A.ochraceus | 2 | 6.7 | 2 | 7.4 | 2 | 9.1 | 1 | 3.4 | 1 | 5.6 | 8 | 6.3 |
| A. terreus | 1 | 3.3 | 1 | 3.7 | 1 | 4.5 | 2 | 6.9 | 2 | 11.1 | 7 | 5.6 |
| Total | 30 | 100 | 27 | 100 | 22 | 100 | 29 | 100 | 18 | 100 | 126 | 100 |
* percentages were calculated in relation to the total number of isolated Aspergillus species of examined samples.
Table 4: Lipolytic and proteolytic activities of the isolated moulds.
| Species | No. of tested | Lipolytic |
|
Proteolytic | ||||||
| Positive | *H | M | W | Positive | H | M | W | |||
| A. flavus | 37 | 37 | 37 | 0 | 0 |
|
30 | 30 | 0 | 0 |
| A. niger | 54 | 49 | 48 | 1 | 0 |
|
48 | 8 | 38 | 2 |
| fumigatus | 11 | 8 | 0 | 3 | 5 |
|
6 | 5 | 1 | 0 |
| parasiticus | 9 | 0 | 0 | 0 | 0 |
|
0 | 0 | 0 | 0 |
| ochraceus | 8 | 5 | 0 | 2 | 3 |
|
5 | 0 | 0 | 5 |
| A. terreus | 7 | 3 | 0 | 0 | 3 |
|
5 | 0 | 0 | 5 |
|
Penicillium sp. |
64 | 64 | 5 | 58 | 1 |
|
64 | 30 | 4 | 30 |
|
Cladosporium sp. |
23 | 23 | 0 | 0 | 23 |
|
18 | 0 | 15 | 3 |
|
Sporotricum sp. |
12 | 8 | 0 | 0 | 8 |
|
5 | 0 | 1 | 4 |
|
Alternania sp. |
12 | 12 | 0 | 0 | 12 |
|
12 | 0 | 0 | 12 |
|
Fusarium sp. |
9 | 8 | 0 | 4 | 4 |
|
7 | 0 | 1 | 6 |
|
Rhizopus sp. |
9 | 9 | 0 | 2 | 7 |
|
9 | 0 | 0 | 9 |
|
Mucor sp. |
7 | 7 | 0 | 1 | 6 |
|
7 | 0 | 0 | 7 |
*High activity (H): more than 11 mm; Moderate (M): 6-10 mm; Weak (W): less than 5 mm.
those obtained by Ouf et al. (2010), Elsayed et al. (2018) and Ashraf et al. (2019) while higher prevalence was recorded by Ali et al. (2005) and El-Tabiy (2006). The results of sausage samples were nearly similar to those obtained by Fatema (2016) and Elsayed et al. (2018) and were lower than those obtained by Brr et al. (2004). The results of the examined minced meat samples are nearly similar to those obtained by Saad et al. (2015) and were lower than those obtained
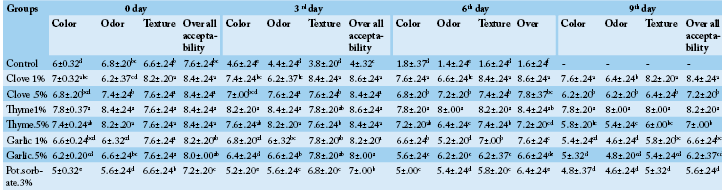
Table 5: Organoleptic evaluation of minced meat samples treated with clove, thyme, garlic (0.5-1%) and potassium sorbate.
a,b,c Different superscripts within each column indicate significant differences (P<0.05).
by El-Tabiy (2006) and Eleiwa and El-Diasty (2014). The results of the examined burger samples were nearly similar to those obtained by Brr et al. (2004). The variation of total mould count in samples may be due to different levels of hygiene during manufacturing and storage. The highest total mould count in sausage may be attributed to the composition of sausage which is minced meat packed in the intestine of animals that may be inadequately cleaned. Concerning the identified mould genera the data obtained from Table 2 as clear in results previously nearly similar to that obtained by Adriana et al. (2002), Mizakova et al. (2002), Brr et al. (2004), Ioannis and Filiousisa (2007) and Seham et al. (2013). These results of proteolytic activity are nearly similar to those obtained by Ahmed and Abdel-Sater (2003), El-Diasty and Salem (2007) who studied the ability of A. niger, Cladosporium spp, A. flavus, Mucor spp and Penicillium spp and they found that all isolates had the ability to produce proteolytic and lipolytic enzyme within different levels (Table 4). The lipolytic activity results, nearly similar to those obtained by Subash et al. (2005), El-Diasty and Salem (2007), Ouf et al. (2010), Shaltout et al. (2014), Abdel-Sater et al. (2017) and in disagreement with Ashraf et al. (2019).
The organoleptic character are very important aspects for meat quality, sometime used in determining the acceptability and the rejection of the products Mottram (1994). Meanwhile, Miller (1994) mentioned that texture and colour of muscle influence acceptability and are the most reliable and rapid indicators of their quality. Organoleptic examination, revealed that, the colour, odour and texture of the control sample changed at 3rd day (Table 4). The sensory changes were attributed to proteolysis and lipid oxidation especially in untreated samples (control) that was more obvious in shorter time than those in treatments due to the progressive growth of microbial load. Spoilage is caused simply due to accumulation of very huge numbers of microbial cells and not related to the metabolic activity of the microbes. Similarly, color changes in meat products can occur due to microbial growth on the surface. The green color of meats, caused by lactic acid bacteria (LAB) (Sperber, 2009). The sensory properties of treated minced beef samples during cold storage (4 ºC) were improved by using the different treatments, and high scores organoleptically from the panel of judges given to thyme 1% treated minced beef samples while odour of clove 1% and garlic1% take fewer scores than thyme 1%. Although a panel of judges still gave high scores till 6th day and 9th day, but the mean of total A. flavus count in clove 0.5%, thyme 0.5%, garlic 1%, garlic 0.5% and potassium sorbate 0.3% increased gradually but still lesser than control at 0 time. Addition of few amounts of preservative or EOs could be a way to maintain a balance between antimicrobial efficacy and sensory acceptability. Food safety attained by using of preservative to control spoilage microorganisms that attack food or making it unsafe. Many food preservation systems include refrigeration, heating and addition of antimicrobial compounds can be used to decrease the rate of food poisoning outbreaks; however, these techniques usually associated with adverse changes in organoleptic characteristics and decrease in nutritional value. Firstly, consumers need higher quality, safe, preservative-free, but mildly prepared foods with long shelflife. New trends are increasingly directed to the possibilities offered by biopreservation (Rasooli, 2007). Minimizing, delaying and inhibiting spoilage and pathogenic organisms are major keys for improving shelf life of fresh meat and increasing human safety (Sallam and Samejima, 2004). The results illustrated in Table 6 showed that samples treated by clove 1% and thyme 1% showed decreases in mean values of total A. flavus through 3rd day and 6th day until 9th day not detected. Clove 0.5%, thyme 0.5%, garlic 1%, garlic 0.5% and potassium sorbate 0.3% showed decreases in mean values of total A. flavus through the 3rd day in which the lowest count recorded, then started gradually but still lower than the count of control in 0 day which explain all treated sample enhanced than the control which have a higher count in 3rd day and give bad scores in organoleptics and decomposed in 6th day. Such findings may be attributed to the high antioxidant effect of clove and thyme EOs, which is related to the scavenger nature of its flavonoids and phenolic content as apigenin, naringenin, luteolin, thymonin, carvacrol and thymol (Senatore, 1996). Velluti et al. (2004) and Lopez et al. (2005) proved antifungal activity of clove oil against filamentous fungi. While many authors Sung et al. (2014), Li et al. (2016), El-Sayed et al. (2017) suggested the use of garlic oil due to its antifungal efficacy. Hussein et al. (2012) recommended the use of Potassium sorbate to improve microbial quality of the meat and increase its shelf-life without any adverse effect on the quality characteristics of the meat.
Table 6: Antifungal activity of various concentrations of essential oils and potassium sorbate against mean count of A. flavus in minced meat samples during cold storage at 4±1°C.
| Groups | 0 day | 3 rd day | 6thday | 9th day |
| Control |
6.12±.07a |
7.53±.07a |
*D | D |
| Clove 1% |
6.06±.06a |
4.52±.17e |
2.83±.43 | ND |
| Clove .5% |
6.12±.07a |
5.11±.11cd |
5.19±.13 | 5.41 ±.13 |
| Thyme 1% |
6.12±.07a |
4.89±.03d |
3.48±.37 | ND |
| Thyme .5% |
6.12±.07a |
5.28±.14bc |
5.39±.13 | 5.63±.07 |
| Garlic 1% |
6.12±.07a |
5.41±.12bc |
5.44±.13 | 5.78±.04 |
| Garlic .5% |
6.12±.07a |
5.60±.04b |
5.64±.04 | 5.90±.02 |
| Pot.sorbate .3% |
6.12±.07a |
5.54±.07b |
5.57±.06 | 5.94±.02 |
a,b,c Different superscripts within each column indicate significant differences (P<0.05); *D:Decomposed.
Conclusion
The result of this study revealed improper hygienic measures during processing. Therefore, strict hygienic measures should be followed during processing. In addition, strong legislations should be taken to produce products of high keeping qualities.
Acknowledgments
We would like to thank assistance and support provided from Food Control Department, Animal Health Research Institute, Zagazig branch, Egypt.
Authors Contribution
All authors contributed equally.
Conflict of interest
No one of the authors has any conflict of interest to clarify.
References





