Advances in Animal and Veterinary Sciences
Research Article
Effect of Xylazine HCl and/or Ketamine HCl on the Tear Production in Clinically Healthy Dogs
Mohammed A.H. Abdelhakiem1*, Enas Elmeligy2, Al-lethie Al-lethie3
1Department of Surgery, Anaesthesiology and Radiology, Faculty of Veterinary Medicine, Assiut University, 71526, Assiut, Egypt; 2Veterinary Teaching Hospital, Faculty of Veterinary Medicine, Assiut University, Assiut 71526, Egypt; 3Department of Surgery, Anaesthesiology and Radiology, Faculty of Veterinary Medicine, Aswan University, Aswan 81528, Egypt.
Abstract | Objective: The effect of commonly used sedative and anesthetic drugs (xylazine and ketamine HCl) on tear production rate was evaluated in dogs using Schirmer tear test-I (STT I). Materials and Methods: Tear production using Schirmer tear strips were measured in healthy dogs before and after intramuscular injection of 1mg/kg of xylazine HCl (X; n = 4), 5 mg/kg of Ketamine HCl (K; n = 4), and combination of xylazine (1mg/kg) and ketamine (5 mg/kg) (XK; n = 4). The tear production was recorded at times 0, 10, 20 and 30 minutes after injection of drugs. Results: Tear production rate significantly decreased (P< 0.05) at 10, 20 and 30 minutes from xylazine injection. It significantly increased (P< 0.05) at 30 minutes elapsed after ketamine injection. In the third group (XK), there was a significant decrease in tear production until 20 minutes after the injection of drugs. The effect of ketamine appeared clearly after 30 minutes to return the STT I values mostly to the pre-treatment values. Conclusion: The study recommends the use of a sterile ocular lubricant or tears replacement as a corneal protectant in dogs sedated or anesthetized using xylazine or a combination of xylazine-ketamine respectively.
Keywords | Dogs, Ketamine, Schirmer tear test-I, Tear production, Xylazine
Received | June 25, 2019; Accepted | September 05, 2019; Published | November 01, 2019
*Correspondence | Mohammed A.H. Abdelhakeim, Department of Surgery, Anaesthesiology and Radiology, Faculty of Veterinary Medicine, Assiut University, 71526, Assiut, Egypt; Email: hamdysurgery@yahoo.com
Citation | Abdelhakiem MAH, Elmeligy E, Al-Lethie A (2019). Effect of xylazine HCl and/or ketamine HCl on the tear production in clinically healthy dogs. Adv. Anim. Vet. Sci. 7(11): 1015-1020.
DOI | http://dx.doi.org/10.17582/journal.aavs/2019/7.11.1015.1020
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Abdelhakeim et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Alpha-2 adrenoreceptor agonists are popular and widely used in small animal practice (Greene, 1999; Murrell and Hellebrekers, 2005). This group includes different members with different affinity to the ὰ-2 receptors. The Medetomidine and xylazine HCl represented the more and less ὰ-2 receptor agonist respectively (Muir et al., 2013). However, xylazine is considered a common agent of the drugs which are used for premedication in some countries. This may be due to its availability relative to other drug members, its low price, and its outstanding sedative and analgesic effects. Moreover, the effect of these drugs is reversible due to the availability of the alpha-2 receptor antagonist (Uilenreef et al., 2008). The practical use of xylazine alone or in combination with other agents may depend upon the purpose of its use. The most common anesthetic drug used in combination with xylazine in small animal practice is ketamine HCl (Haskins et al., 1986; Hellyer, 1996; Kul et al., 2000). There are several studies which did not recommend the use of xylazine or xylazine combination in small animal practice due to its outstanding adverse effects. The anesthetic regime in small animals, including xyalzine, was associated with a high mortality rate and anesthetic risk (Clarke and Hall, 1990; Dodman & Lamb, 1992; Sinclair, 2003). The effects of xylazine and/or ketamine on different body systems were reported in the literature (Muir et al., 2013). The use of xylazine and ketamine for sedation and general anesthesia for ophthalmic surgeries was recorded in a few studies (Idvall et al., 1979; Hazra et al., 2008). It’s well-known that the precorneal tear film has an important role in the nourishment of the non-vascularized cornea. It maintains the integrity of the surface of the globe (Samuelson, 1991). Due to most of the anesthetic regimes associated with a decrease of the tear production which may lead to micro-injuries of the ocular surface (Drupin, 1977; Pontes et al., 2010), The selection of drugs have no or little effect on tear production of the animals subjected to surgical operations is so important. Little information about the tear production after xylazine and/ or ketamine administration in dogs was published (Dodam et al., 1998; Muir et al., 2013; Kanda et al., 2016). As well as the results of the previous studies regarding the effect of xylazine on tear production in different species are somewhat contradicted (Brightman et al., 1983; Dodam et al., 1998; Ghaffari et al., 2010; Kanda et al., 2016, 2019).
To our knowledge, the effect of ketamine HCl alone or in combination with xylazine HCl on the tear production in healthy dogs was not assessed. So, the purpose of this study was to investigate the effect of xylazine, ketamine and their combination on the tear production in dogs using Schirmer tear test I (STT I).
MATERIALS AND METHODS
Animals
The study was carried out on four adult intact clinically healthy mongrel dogs (3 males and one female). Their ages ranged from 2 to 3 years and their weight from 12 to 19 kg.
Pre-Treatment Preparation of the Animals
The animals were enclosed in a well-equipped room in the animal hospital, Assiut University. The food and water were provided to animals in a continuous manner. The dogs were dewormed using (praziquantel 5 mg/kg p.o.- Epico company, Egypt) and (Ivermectin 0.2 mg/kg/week for 4 weeks- Merial company, USA). Dogs were allowed to acclimatize to their new environment for a period of 3 weeks before the beginning of the study. Once they accommodated the place and individuals, the study was carried out.
Each animal underwent a clinical and ocular examination. The dogs were examined thoroughly. Their eyes were normal and free from any lesions (The conjunctivae were rosy red, free from injected blood vessels, and no ocular discharge). The temperature (Temp), heart rate (HR), and respiratory rate (RR) were evaluated before and after the beginning of treatments.
Study Design
The dogs were subjected to three treatments with 14 days interval between them. The first treatment (X), the animals received 1mg/ kg of xylazine HCl. The second one (K), they received 5 mg/kg of ketamine HCl, and the third treatment (XK), the dogs were injected with the same doses of xylazine (1mg/kg) and ketamine HCl (5mg/kg). All injections were carried out through the intramuscular route.
The tear reading using Schirmer tear strip (OpStrip, Ophtechnics unlimited Co, Haryana, India) was taken from each animal (right eye) before each treatment and every 10 minutes after injection of drugs. The readings of Schirmer were recorded until 30 minutes post-injections. The end of the measuring paper strip was placed close to the lateral canthus in the conjunctival sac between the lateral lower eyelid and the cornea. The strips were removed after 1 min and the value recorded and tabulated immediately. The physical parameters (Temp, HR, and RR) also were recorded every 10 minutes until 30 minutes post-administration of sedative and/or anesthetic.
Statistical analysis
Data were presented as mean and standard error (mean± SE). The data were analyzed using statistical package for the Social Sciences for Windows (SPSS, version 21 (2016), Chicago, IL, USA). The Friedman non-parametric test for repeated measures was used to evaluate the means of different groups. Once the significant difference was found, a repeated measure ANOVA was used to study the change of Schirmer tear readings and physical signs (temperature, HR and RR) at 10, 20, and 30 minutes post-injection of drugs to the pre-treatment values (time zero). Differences were considered significant when P < 0.05.
RESULTS
The values of Schirmer tear strips before and after different treatments were summarized in Table 1 and Figure 1.
Table 1: Schirmer tear readings (mm) in different groups
| Time |
Xylazine (mean± SE) |
Ketamine (mean± SE) |
Xylazine-ketamine (mean± SE) |
| Before (B0) |
16± 1.1a |
14.75± 0.48a |
16.75± 1.2a |
| 10 minutes after |
4.75± 0.85a,b |
15.25± 0.75b |
8.25± 1.11a,b |
| 20 minutes after |
1.25± 0.25a,b |
16.5± 0.96c |
9.25± 0.95a,c |
| 30 minutes after |
3± 0.91a |
18.5± 0.87a,b,c |
12.75± 0.85b,c |
All the data are expressed in mean± standard error (SE)
There are significant variations between the values which were superscripted by the same letter.
The pre-treatment values of Schirmer tear test ranged from 14-20 mm/minute in all groups. There were significant decreases of STT I values after xylazine injection along different intervals. According to the ketamine group, there were non-significant increases of the STT I value at the first two intervals (10 and 20 minutes), but at 30 minutes post-injection of ketamine it significantly increased relative to the pre-treatment value. In the combination group, the effect of xylazine was clear than the ketamine effect. There were significant decreases of the STT I values at 10 and 20 minutes post-injection. At 30 minutes post-injection of drugs the ketamine effect was outstanding to return the STT I value to mostly the pre-treatment value.
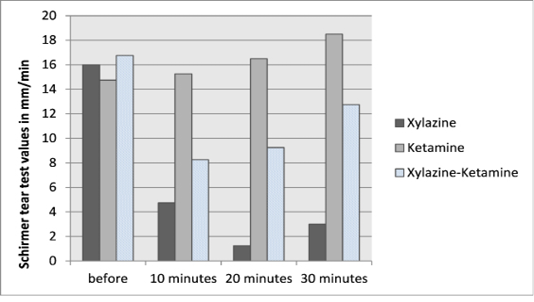
Figure 1: Baseline (before) and post-injection Schirmer tear test I values for dogs treated with xylazine HCl, ketamine HCl and xylazine-ketamine combination.
Table 2: Physical data of xylazine HCl, ketamine HCl, and xylazine-ketamine combination
| Factor and time | Xylazine (Mean± SE) | Ketamine (Mean± SE) | Xylazine-ketamine (Mean± SE) |
| Temp 0 |
38.88± 0.33A |
38.88± 0.33 | 38.93± 0.3 |
| Temp 10 |
39.05± 0.46B |
39.65± 0.22 |
39.3± 0.38A |
| Temp 20 |
39.4± 0.46A,B |
39.58± 0.2 |
38.93± 0.38A,B |
| Temp 30 |
39.4± 0.5B |
39.83± 0.23 |
38.7± 0.39A,B
|
| HR 0 |
62.5± 2.99A |
62.5± 2.99A |
77.5± 13.15 |
| HR 10 |
35.25± 2.3A,B |
117.5± 2.5A |
71.5± 5.7 |
| HR 20 |
43.75± 2.4A,B |
110± 5.8A |
59± 5.92 |
| HR 30 | 45.75± 4.8 | 95± 9.6 |
61.5± 2.99
|
| RR 0 |
21.5± 2.99A |
21.5± 2.99A |
31± 5.75 |
| RR 10 |
9± 1.3A |
65± 5A |
18.5± 2.5 |
| RR 20 |
10± 0.82A |
60.5± 14.4A |
17.5± 3.5 |
| RR 30 |
8.75± 1.8A |
47.5±6.3A |
21.5± 2.99 |
Legend: Temp= temperature, HR= heart rate, RR= respiratory rate.
All the data are expressed in mean± standard error (SE)
There are significant variations between the values which were superscripted by the same letter.
According to the physical parameters, there were significant increases and decreases of temperature after injection of xylazine HCl and xylazine-ketamine combination respectively. The ketamine injection alone did not affect the body temperature. There were significant decreases of both heart rate and respiratory rate after xylazine injection. However, the ketamine injection caused significant rises of the heart rate and respiratory rate. The xylazine-ketamine combination had a neutral effect on both HR and RR, causing non-significant changes (Table 2).
DISCUSSION
It’s well-known that the pre-corneal tear film has different functions. One of them is the nourishment of the cornea. The surface of the eye is rapidly deteriorated in cases of decrease of tear production such as dry eye or keratoconjunctivitis sicca (Alkan et al., 2004; Chandler et al., 2013). Due to the most of anesthetic regimes are associated with changes of volume of tear. So, this study was conducted to investigate the effect of most common sedative and anesthetic drugs in Egypt on the tear production in dogs. The Schirmer tear test I (STT I) is a semiquantitave method used without topical anesthesia for determination of the baseline reflex secretion (Berger & King, 1998; Alkan et al., 2004).
According to the results of the present study, the Schirmer tear test I (STT I) values before the injection of drugs in different groups ranged from 14-20 mm/min. The previous studies reported the normal range of the Schirmer tear test in dogs ranged from 14-25 mm/min (Gelatt et al., 1975; Kaswan & Salisbury, 1990). The wide range may be due to several factors such as sex, breed, measurement sequences, daily time and light cycle (Alkan et al., 2004; Gianetto et al., 2009).
The drugs of this study were selected because they are generally used by veterinarians due to its availability and lower cost relative to other sedative and anesthetics. Xylazine HCl is considered the most common sedative drug used for different animals in Egypt. The effect of xylazine either alone or in combination with other drugs was studied before. Dodam et al. (1998), investigated its effect on tear production in dogs. They found xylazine HCl (0.5 mg/kg) alone did not affect the tear production, but when it combined with butarphanol (0.5mg + 0.5mg/kg) significantly decreased the tear production. The same result was reported in horse by Brightman et al. (1983). The results of the present study were not in consistent with the results of the previous studies. In the present study, xylazine HCl profoundly decreased the tear production in dogs which continued until 30 minutes post-injection. Also, this effect was clearly noted in the combination group (XK). The effect of xylazine persisted until 20 minutes after injection of ketamine HCl. The latter could not mask the effect of xylazine. The results of this study differed substantially from the previous studies. This may be due to the difference of xylazine doses which used in different studies. This attribution was confirmed by Kanda et al. (2016). They recorded the effect of 0.5 mg/kg of xylazine appeared after 30 minutes elapsed after its intramuscular injection in dogs. Moreover, the sensitivity of the different species to xylazine varied. The equine is less sensitive to xylazine effects compared to other species (Hall et al., 2001). In the present study, 1mg/kg of xylazine was used for sedation of dogs. Hall et al. (2001), and Kanda et al. (2016) reported the dose dependent effect of xylazine in animals. Thus, the increase of dose may increase the drug effect including the significant decrease of tear production. Also, the different results may be attributed to the unknown mechanism of action of xylazine. It was described as excitatory and inhibitory for both adrenergic and cholinergic neurons (Kronberg et al., 1966). On the other side, the results of xylazine in this study were in accordance with the results reported by Ghaffari et al. (2010, 2017) in cats and horses and Kanda et al. (2016, 2019) in dogs and cats. They recorded the decrease of tear production after injection of different doses of ὰ-2 agonists either medetomidine HCl or xylazine HCl in dogs. Although the results of the present study were consistent with the previous studies that xyalzine reduces the tear production profoundly, the difference was in the beginning time of this reduction. The present study revealed that significant decrease started 10 minutes and persisted to 30 minutes after injection of 1mg/kg of xyalzine. But, the study conducted on dogs by Kanada et al. (2016) reported the significant effect of 1mg/kg of xyalzine on tear production appeared after 0.5 hour from its injection. Actually the duration effect of xyalzine on tear production was not the aim of our study. Therefore, the effect of xylazine was not traced for long time.
Kanada et al. (2016) verified that the effect of xylazine on tear production is mediated through α2-adrenoceptor action and excluded effects on α1-adrenoceptors or imidazoline receptors. As well as, Komnenou et al. (2013), reported the adrenergic innervation of lacrimal glands in mice not in dogs. This means that the xylazine effect on tear production in dogs does not occur through the adrenergic receptors. The exact mechanism beyond the decrease of tear production after xylazine administration is not fully elucidated. But the suggested acceptable mechanism was the central effect of ὰ-2 agonists on the autonomic regulation of tear production. Additionally, it was recorded the vasoconstriction of the tear gland, alteration of the metabolism of its cells and suppression of reflex tear production through increased antinociception (Dodam et al., 1998; Sanchez et al., 2006; Komnenou et al., 2013). It is postulated that the decrease of tear flow may be attributed to reduction or inhibition of the parasymapathatic innervation to the tear gland by the xylazine through the central effect.
On the other hand, the present study revealed the significant increase of tear production in dogs after intramuscular injection of 5mg/kg of ketamine HCl. There were previous studies which suggested the decrease of tear production after using a combination of midazolam, ketamine and isoflurane in rabbits (Erol et al., 2018), and ketamine and acetylpromazine combination in atropine premedicated cats (Arnett et al., 1984). There was no previous study investigated the effect of ketamine as a sole agent on the tear production in dogs. The effect of ketamine in the present study was assessed alone and in combination with xyalzine HCl. In both treatments the significant effect of ketamine to increase the tear production or the values of STT I was noticed. The secretory effect of the lacrimal gland is mediated through the parasympathetic innervation conveyed through the cranial nerves V (ophthalmic and maxillary divisions), VII and pteropalatine ganglion (Garosi, 2012). The increase of tear production after ketamine injection indicates that the parasympathetic innervation to the tear gland was not blocked but stimulated. So, the authors postulated that the stimulatory effect of ketamine on tear flow might be ascribed to the ketamine is a dissociative anesthetic which may affect regions in brain and does not affect others. The nuclei of CNs V and VII may be in the regions away from ketamine effect. As well as, the increase of cardiac output, mean aortic pressure, pulmonary arterial pressure, central venous pressure, and heart rate after ketamine injection (Haskins et al., 1985) may increase the blood flow into different tissue including the tear gland.
In the group received xylazine and ketamine, there was a significant decrease of the tears after administration of xylazine which lasted to about 20 minutes after ketamine injection, then returned nearly to the normal values or the pre-anesthetic values after 30 minutes of ketamine and xylazine administration. This effect was nearly similar to the effect resulted from medtomidine-ketamine combination in cats (Di Pietro et al., 2016).
Regarding the physical signs, xylazine HCl caused significant increases in body temperature and significant decreases in both heart and respiratory rates (Table 2). These results were consistent with the results of the previous studies (Kolliasbaker et al., 1993; Pypendop & Verstegen, 1998; Sinclair, 2003; Lerche & Muir, 2004; Posner, 2018a). The increase in body temperature may be attributed to the peripheral vasoconstriction caused by ὰ-2 agonists. The decrease in heart rate may be due to the decrease of the release of norepinephrine and the heart is influenced mainly by the parasympathetic action (Posner, 2018a). It was reported that the decrease in respiratory rate after xylazine injection is centrally mediated (Kolliasbaker et al., 1993; Pypendop & Verstegen, 1998; Sinclair, 2003; Lerche and Muir, 2004).
Ketamine HCl did not affect the body temperature, but it significantly increased both the heart and respiratory rates. These results were in agreement with what was recorded previously by Haskins et al. (1985) and Posner (2018b). It increases the heart rate through the direct stimulation of the central adrenergic centers (i.e., increased sympathetic tone) and by inhibiting the neuronal uptake of catecholamines, especially norepinephrine (Annetta et al., 2005). Ketamine causes a moderate increase in carbon dioxide concentrations, so it considered respiratory stimulants, unlike other anesthetics which lead to respiratory depression and apnea (Posner, 2018b).
The addition of the respiratory depressant such as xylazine HCl with ketamine leads to the decrease of the respiratory rate and minute volume (Posner, 2018b). In the third group, there were non-significant decreases in the heart and respiratory rates. Therefore, this combination is so beneficial and safe to the patient. But the body temperature significantly decreased at 20 and 30 minutes post-injection of drugs relative to the body temperature at 10 minutes post-injection. These results were in agreement with Afshar et al. (2005) in goats. But the previous studies conducted by Kul et al. (2000) and Wyatt et al. (1989) did not show any significant changes in the body temperature in dogs and rabbits after xylazine-ketamine injection. The decrease of body temperature could not be elucidated, but the authors expected that the recumbency of animals due to the effect of the combined two drugs which might have the responsibility for the decrease in body temperature (Abdelhakiem et al., 2019).
CONCLUSION
It could be concluded from the present study that the xylazine and ketamine HCl causing a significant decrease and increase of tear production respectively when each used alone, but when they combined the effect will not be severe to cause detrimental action on the surface of the eye. It is preferred that both of the xylazine and ketamine will be in the same syringe at the time of injection or at least injected at the same time without an interval between them. Moreover, the dose of xylazine should be lower than 1 mg/kg to get acceptable results. The authors recommended the use of eye lubricants, or artificial tears when xylazine alone or xylazine- ketamine combination used in dogs.
ACKNOWLEDGMENTS
The authors would like to thank the head department of Animal Surgery, Anesthesiology and Radiology; Faculty of Veterinary Medicine, Assiut University for approval of carrying out this study and his support.
CONFLICT OF INTERESTS
The authors declare no conflict of interest.
AUTHORS’ CONTRIBUTON
Dr. Mohammed Ahmed Hamdy Abdelhakiem, placed the study design, participated in carrying out the study, analyzed the data statistically, wrote the manuscript, formatted the manuscript according the journal style and finally submitted it. Dr. Al-lethie Alhaz Al-lethie, had the idea of the study, participated in carrying out the study, arranged the data in tables, revised the manuscript. Dr. Enas Elmeligy, participated in carrying out the study. All the authors read and finally approved the manuscript for publication.
REFERENCES





