Advances in Animal and Veterinary Sciences
Research Article
Palliative Combined System Immune Chemotherapy with Ligfol and Futoruracyl of Mammary Gland Cancer Relapse in Incurable Cases
Vladimir Vasilyevich Salautin*, Vitaly Ivanovich Gorinsky, Grigory Prokofievich Demkin, Alexey Vyacheslavovich Molchanov, Nikolai Aleksandrovich Pudovkin, Ivan Yurevich Domnitsky, Svetlana Evgenevna Salautina
Saratov State Agrarian University named after, Saratov, st. Sokolovaya 335, (8452) 69-25-31, N.I. Vavilova.
Abstract | The aim of the study was to neutralize the toxic effect of chemotherapy, with simultaneous activation of immunological reactions mediated by T and B lymphocytes, natural killers and macrophages. When conducting a set of diagnostic measures, the anamnestic data, the results of physical examination, electrocardiographic, ultrasound and X-ray studies were taken into account. The article presents the results of studies on the effect of palliative combined system immune chemotherapy with Ligfol and Fluorouracil on mammary gland cancer relapse in dogs in incurable cases. Hematological and biochemical blood tests and urinalysis were performed in all animals. For the classification of malignant neoplasms, tumor, nodus and metastasis (TNM) classification was modified, taking into account specific features of dogs. It has been established that a course of the combined systemic immuno-chemotherapy with Ligfol and Fluorouracil in females with recurrent mammary gland cancer, according to our proposed scheme, has low overall and hematological toxicity, which does not require delay in treatment, reduction of the dose of chemotherapy drug, and corrective therapy. In addition, the proposed treatment strategy allows achieving stabilization of the tumor process.
Keywords | Mammary gland cancer, Chemotherapy, Ligfol, Fluorouracil, WBCs, RBCs, Platelets
Received | June 12, 2019; Accepted | August 30, 2019; Published | October 15, 2019
*Correspondence | Vladimir Vasilyevich Salautin, Saratov State Agrarian University named after, Saratov, st. Sokolovaya 335, (8452) 69-25-31, N.I. Vavilova; Email: salautin60@mail.ru
Citation | Salautin VV, Gorinsky VI, Demkin GP, Molchanov AV, Pudovkin NA, Domnitsky IY, Salautina SE (2019). Saratov State Agrarian University named after, Saratov, st. Sokolovaya 335, (8452) 69-25-31, N.I. Vavilova. Adv. Anim. Vet. Sci. 7(s1): 33-39.
DOI | http://dx.doi.org/10.17582/journal.aavs/2019/7.s1.33.39
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Salautin et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Oncological diseases of small domestic animals are among the most topical problems of the modern veterinary medicine. One of the main conditions for solving this problem is to clarify etiopathogenesis and the prevalence of cancer among domestic dogs (Salautin et al., 2018).
The value of drug antitumor therapy, as an independent method or as a stage of complex treatment, is indisputable. Cytotoxic drugs are invariably used both in neoadjuvant and in adjuvant regimens, as well as in cases when other methods of treatment cannot be applied (Tyulyandin et al., 2010). Fluorouracil is highly toxic substance. It belongs to the group of antimetabolites. Its pharmacological action is related to disruption of DNA synthesis and the formation of structurally imperfect RNA, which inhibits the division of tumor cells. The main indication for the use of fluorouracil is drug therapy of various types of malignant tumors, often incurable cases, including mammary gland carcinoma. The drug is used both independently and as part of polychemotherapy. In the course of therapy, despite the careful selection of the dosing regimen, its toxic effect on the oral mucosa, digestive system, kidneys, hematopoietic organs, skin, and nervous system is often manifested. Clinically, the drug effect is manifested by stomatitis, anorexia, vomiting, diarrhea, gastrointestinal bleeding, leukopenia and thrombocytopenia, the appearance of alopecia, dermatitis and cerebellar ataxia. In some cases, hyperthermia, septicemia, pharyngitis and pneumonia can be seen (Gorinsky et al., 2018; Trofimtsov et al., 2017; White, 2016; Withrow et al., 2013). Side effects of anticancer drugs are characterized by the ability to have a damaging effect not only on tumor cells, but also on actively proliferating “healthy” cells of the body. Thus, these side effects are the limiting factor for chemotherapy. Fluorouracil may have a side effect on the animal’s organism at different times after its application – directly at the stage of chemotherapy, immediately after its termination or later. In this regard, it is important to improve the methods used to prevent, attenuate and eliminate the negative impact of antitumor therapy on the organs and systems of the body. This, in turn, will considerably expand the introduction of chemotherapy into clinical practice.
The aim of the study was to study the effect of Ligfola and Fluorourocil on the neutralization of the toxic effect of chemotherapy, activation of immunological reactions mediated by T and B lymphocytes and macrophages.
MATERIALS AND METHODS
Doggess of different breeds and ages served as material for the study suffering from mammary gland cancer relapse. Clinical observations and studies were carried out at the veterinary clinic of the Center of Beauty and Animal Health Zoostil of the sole proprietor Gorinsky V.I. (Volgograd) and the Department of Morphology, Animal Pathology and Biology of the Federal State Budgetary Educational Institution of Higher Education Saratov State Agrarian University named after N.I. Vavilov.
The anamnestic data, the results of physical examination, electrocardiographic study, ultrasound examination, X-ray studies were taken into account when conducting a complex of diagnostic measures. Hematological and biochemical blood tests and urinalysis were performed in all animals. Modified TNM classification was used for the classification of malignant neoplasms taking into account specific features of the dogs.
Total number of animals was 7 (n = 7). According to the scheme developed and proposed by the authors, all the studied animals were given a combined systemic immune chemotherapy with Ligfol and Fluorouracil. Pharmacological properties of Ligfol were based on the ability of humic acids, being part of the drug to stimulate immune-antioxidant mechanisms of the body, increasing nonspecific immunity and resistance of the animal to adverse factors (Gorinsky and Salautin, 2016; Gorinsky et al., 2018). Thus, this fact determined the choice of the drug in our study.
Characteristics of the therapy:
1. Fluorouracil – i.v., at the dose of 200 mg/m2, one time every 7 days, 6 courses.
2. Ligfol – i.m., at the dose of 0.1 ml per 1 kg of animal weight to females weighing up to 10 kg, and 1 ml per animals for those with a mass of ≥ 10 kg–once every 3 days for 6 weeks, in parallel with the use of Fluorouracil. The first dose of Ligfol was administered 24 hours before the start of chemotherapy.
The WHO criteria were used in order to determine the degree of side effect, anticancer therapy, hematologic toxicity and assess the effectiveness of the therapy.
RESULTS AND DISCUSSION
Ligfol and Fluorouracil were administered to the following patients. Patient 1. Doggess, Miniature Schnauzer breed, at the age of 10 years, 8.8 kg, not sterilized, ruttishness was every 6 months, last gestation and parturation were about 3 years ago. For the whole period of life, there were 4 gestations. Anamnesis morbi: localization of the primary M1 tumor on the right side, T2N0M0. Simple mastectomy was performed in the animal. The histological diagnosis was the following: carcinoma of the mammary gland, grade I malignancy, adjuvant chemotherapy (chemotherapy) was not performed. After 6 months, relapse was detected. The results of the clinical study were the following: localization of M2 tumor nodes (Figure 1) and M4 on the right side, T3N2/T2N0, distant metastases were not detected. The results of the hematological studies were the following: WBC 9.4 x 109/L; Lymph 2.1 x 109/L; Mon 0.3 x 109/L; Gran 7.0 x 109/L; Lymph % 22.4; Mon % 3.5; Gran % 74.1; Eos % 4.7; RBC 7.31 x 10 12/L; HGB 152 g/L; HCT % 51.5; PLT 516 x 109/L. The concomitant diseases were the following: cholecystitis and CKD.
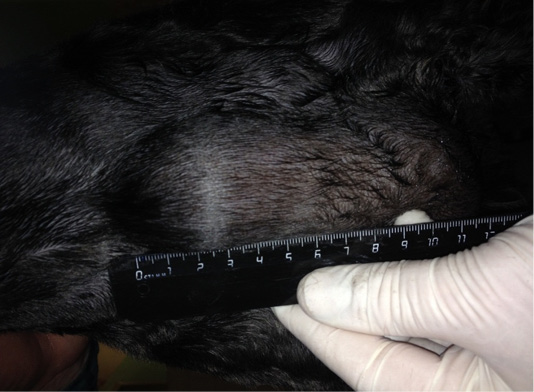
Figure 1: Neoplasm of the mammary gland in the M2 region on the right side. Doggess, miniature schnauzer breed, at the age of 10 years.
Patient 2. Doggess, standard dachshund breed, at the age of 10.5 years, with the weight of 7.5 kg, OGE was 2 years ago due to pyometra. Neither gestations, nor parturation episodes were during the whole period of life. Anamnesis morbi: primary tumor in M4 region on the right side, T2N0M0. Simple mastectomy was conducted in the animal. The histological diagnosis was the following: infiltrating adenocarcinoma of the mammary gland, grade I of malignancy, with a stromal reaction. Adjuvant CT was not performed in the animal. Relapse was noted after 12 months. The results of the clinical study were the following: localization of tumor nodes: M4 and M5 on the left side, T2N0/T2N1 (Figure 2); M1 and M5 on the right side T2N1/T2N2. Distant metastases were not detected during the primary admission. The hematological indicators were the following: WBC 7.6 x 109/L; Lymph 2.0 x 109/L; Mon 0.4 x 109/L; Gran 5.2 x 109/L; Lymph % 26.7; Mon % 4.9; Gran % 68.4; Eos % 0.4; RBC 8.32 x 1012/L; HGB 179 g/L; HCT % 61.5; PLT 343 x 109/L. The concomitant diseases were the following ones: mitral valve endocardiosis, HCM and CKD.
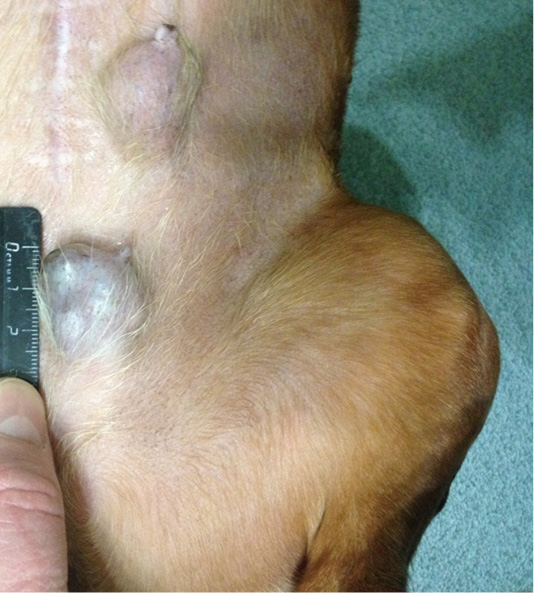
Figure 2: Localization of tumor nodules: M4 and M5 region on the left side. Doggess, breed dachshund standard, at the age of 10.5 years.
Patient 3. Doggess, breed jagdterrier, at the age of 12 years, with the weight of 10 kg, sterilized. There were no gestation and parturation episodes. Anamnesis morbi: primary M1 tumor on the right side, T2N0M0. Simple mastectomy was performed. The histological diagnosis was the following: carcinoma of the mammary gland mixed structure. No adjuvant chemotherapy was performed in the animal; relapse was detected after 7 months. The results of the clinical study were the following: localization of the tumor site at the suture site of the performed mastectomy (Figure 3), T3N2M0. The hematological indicators were the following: WBC 8.7 x 109/L; Lymph 1.9 x 109/L; Mon 0.4 x 109/L; Gran 6.4 x 109/L; Lymph % 21.8; Mon % 4.7; Gran % 73.5; Eos % 1.6; RBC 9.05 x 1012/L; HGB 193 g/L; HCT % 57.7; PLT 684 x 109/L. The concomitant diseases were the following ones: EHR, cholecystitis and CKD.
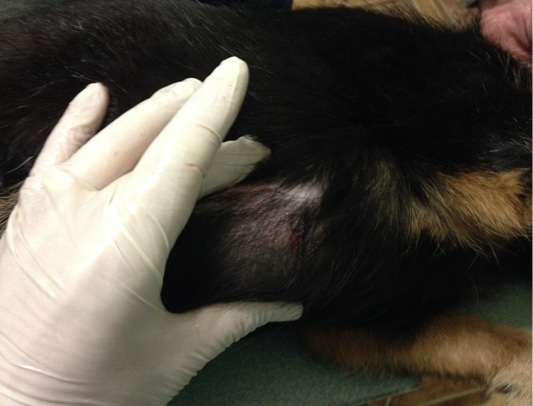
Figure 3: Mammary gland neoplasm in the M1 region on the right side. Doggess, breed jagdterrier, at the age of 12 years.
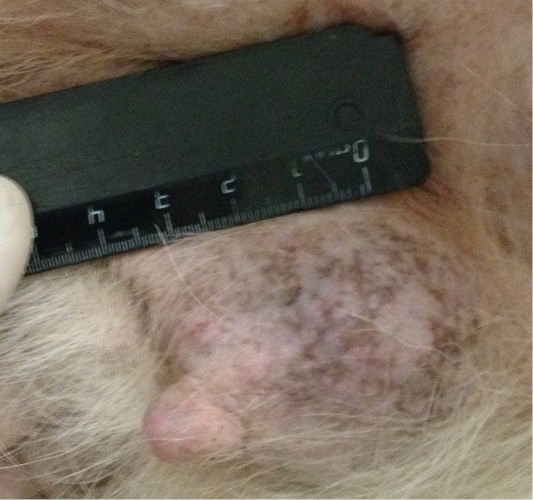
Figure 4: Mammary gland neoplasm in the M4 region on the left side. Doggess, American Bulldog, at the age of 11.5 years.
Patient 4. Doggess, American Bulldog, at the age of 11.5 years, with the weight of 37.2 kg, not sterilized, ruttishness was every 4–6 months. One episode of gestation and parturation was at the age of 4 years. Anamnesis morbi: primary tumor at M5 region on the left side, T3N1M0. Simple mastectomy was performed in the animal. The histological diagnosis was the following: infiltrative ductal carcinoma. Adjuvant CT was not performed. Relapse was detected after 6.5 months. The results of the clinical study were the following: localization of tumor sites in M4 and M5 regions on the right side, T2N0/T2N2; M4 on the left side–T2N0 (Figure 4). The ultrasound study revealed distant metastases in the liver (Figure 5). The hematological indicators were the following: WBC 9.6 x 109/L; Lymph 1.5 x 109/L; Mon 0.3 x 109/L; Gran 7.8 x 109/L; Lymph % 15.9; Mon % 3.6; Gran % 80.5; Eos % 1.9; RBC 6.84 x 1012/L; HGB 147 g/L; HCT % 52.7; PLT 735 x 109/L. The concomitant diseases were the following ones: DCM, CKD, and recurrent eczema.
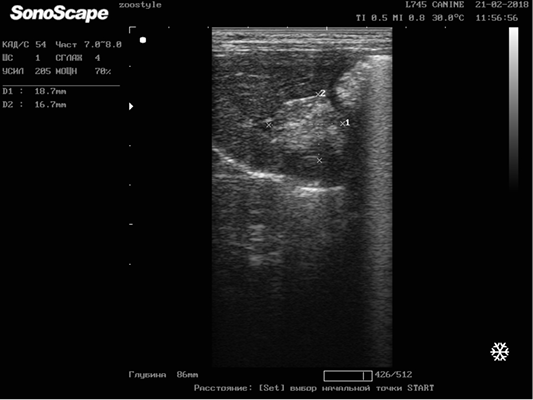
Figure 5: Metastasis of breast cancer in the liver parenchyma. Doggess, breed American Bulldog, at the age of 11.5 years.
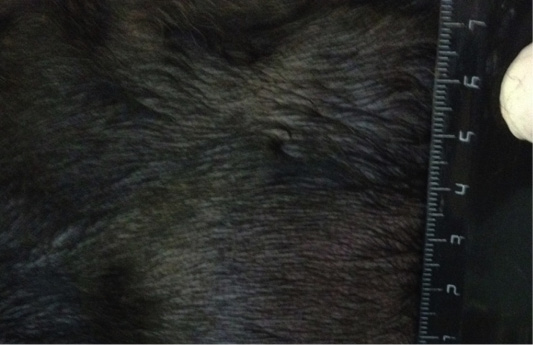
Figure 6: Localization of the tumor in M4 region on the left side. Doggess, Miniature Schnauzer, at the age of 10.5 years.
Patient 5. Doggess, miniature schnauzer, at the age of 10.5 years, 8.5 kg, not sterilized, ruttishness was every 6 months. There were 2 episodes of gestation and parturation, moreover, the last one occurred about 7 years ago. Anamnesis morbi: primary tumor in M5 region on the left side, T2N0M0. Patient underwent a simple mastecomy. Histological diagnosis of moderately differentiated non-infiltrating lobular mammary gland cancer. Adjuvant chemotherapy was not performed in the patient. Relapse was detected after 12 months. The results of the clinical study were the following: localization of the tumor in M4 region on the left side (Figure 6), T3N1M1. Distant metastases were detected on X-Ray in lungs–focuses up to 0.5 cm in diameter (Figure 7). The hematological indicators were the following: WBC 9.9 x 109/L; Lymph 3.0 x 109/L; Mon 0.4 x 109/L; Gran 6.5 x 109/L; Lymph % 30.3; Mon % 4.1; Gran % 65.6; Eos % 1.6; RBC 6.42 x 1012/L; HGB 134 g/L; HCT % 46.1; PLT 523 x 109/L. The concomitant diseases were the following ones: on ultrasonography – diffuse changes in the parenchyma of both kidneys and urolithiasis.
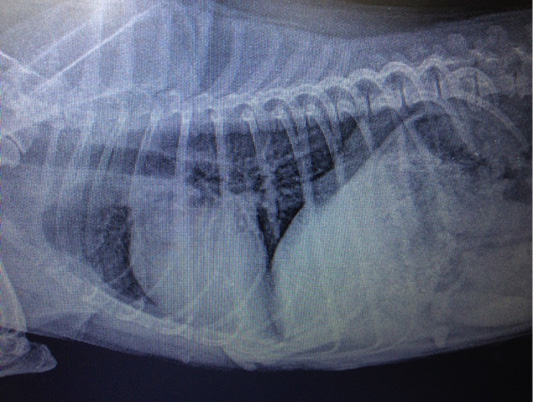
Figure 7: Metastasis of mammary gland cancer in the lungs. Doggess, Miniature Schnauzer, at the age of 10.5 years.
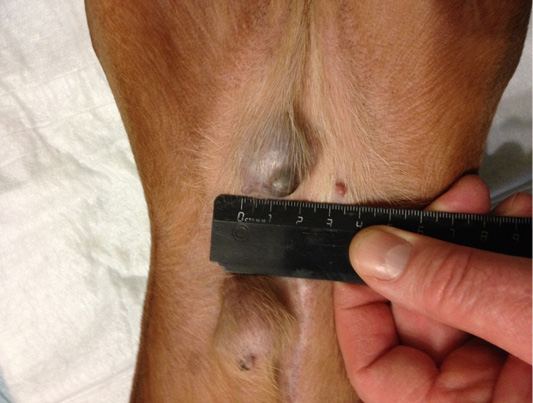
Figure 8: Localization of tumor nodules in M4 and M5 regions on the right side. Doggess, dachshund standard, at the age of 9 years.
Patient 6. Doggess, standard dachshund, at the age of 9 years, 7.8 kg, not sterilized, ruttishness was every 6 months. There were no episodes of gestation and parturation during the whole period of life. Anamnesis morbi: primary M3 tumor
Table 1: Dynamics of hematological indicators.
| Parameters | Before therapy | Day 7 | Day 14 | Day 21 | Day 28 | Day 35 |
| White blood cells x 109/L | 9.51±0.52 | 9.49±0.91 | 8.06±0.36 * | 9.91±1.17 | 8.56±1.99 * | 8.6±0.90 * |
| Granulocytes x 109/L | 6.94±0.48 | 6.39±0.31 | 5.91±0.25 * | 6.90±0.80 | 6.98±0.90 | 6.22±0.82 |
| Hemoglobin g/L | 162.57±8.11 | 162.43±9.92 | 159.29±2.92 | 160.29±4.87 | 164.14±6.78 | 160.0±5.81 |
| Platelets x 109/L | 535.86 ± 57.3 | 487.86±48.72* | 526.14±61.66 | 555.57±73.77 | 535±68.84 | 542.86±51.35 |
Note: significance of differences with respect to control: * –p≤0.05
on the right side, T2N0M0. Animal underwent simple mastectomy. The histological diagnosis was the following: lobular infiltrative cancer. Adjuvant CT was not performed in the animal. Relapse was observed after 3.5 months. The results of the clinical study were the following: localization of tumor nodes in M4 and M5 regions on the right side, T2N0/T2N1 (Figure 8). During the primary admission distant metastases were not detected. The hematological indicators were the following: WBC 12.1 x 109/L; Lymph 2.4 x 109/L; Mon 0.5 x 109/L; Gran 9.2 x 109/L; Lymph % 20.0; Mon % 3.8; Gran % 76.2; Eos % 6.1; RBC 7.89 x 1012/L; HGB 153 g/L; HCT % 54.0; PLT 600 x 109/L. The concomitant diseases were the following ones: mitral valve endocardiosis, left atrial dilation and left ventricular hypertrophy of the heart, CKD.
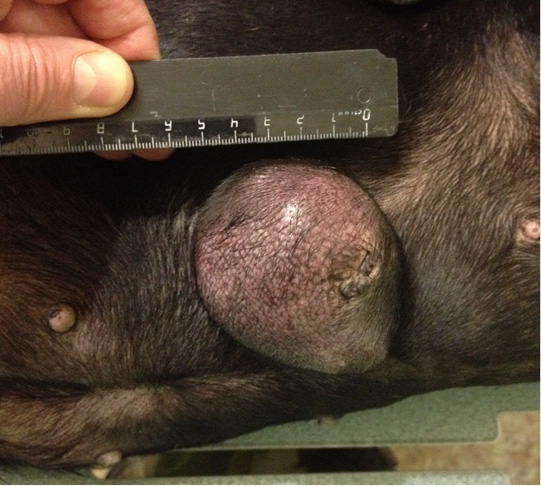
Figure 9: Localization of the tumor site M2 on the right, in the area of the mastectomy previously performed. Doggess, standard dachshund breed, age of 11.5 years.
Patient 7. Doggess, standard dachshund, at the age of 11.5 years, 10.5 kg, not sterilized. Ruttishness was every 6 –7 months. There were no episodes of gestation and parturation during the whole period of life. Anamnesis morbi: primary M2 tumor on the right side, T1N0M0. Simple mastectomy was performed in the animal. The histological diagnosis was the following: infiltrative ductal carcinoma. Adjuvant CT was not performed in the animal. Relapse was observed after 5.5 months. The results of the clinical study were the following: localization of the tumor in M2 region on the right side, in the area of previous mastectomy, T3N1M0 (Figure 9). Distant metastases were not detected during the primary admission. The hematological indicators were the following: WBC 9.3 x 109/L; Lymph 2.4 x 109/L; Mon 0.4 x 109/L; Gran 6.5 x 109/L; Lymph % 25.3; Mon % 4.7; Gran % 70.0; Eos % 1.0; RBC 8.84 x 1012/L; HGB 180 g/L; HCT % 64.7; PLT 350 x 109/L. The concomitant diseases were the following ones: mitral valve endocardiosis, HCM, CKD, overweight.
General differential blood count was performed in all the studied animals with counting of platelets, before the beginning of the treatment, and before each course of chemotherapy (Table 1).
It has been established that before the beginning of the treatment, no leukopenia was diagnosed in any animals. On days 7 and 14 a decrease in the number of WBCs was noted by 0.2 % and 15.3 %, respectively, of the initial value. By day 21, the number of WBCs was restored, and even slightly exceeded the figures of day 1–by 4.2 %. On day 28, a decrease in the number of WBCs was observed again – by 10 %. By day 35 of the study, the number of WBC increased. However, it was 9.6 % lower than before the beginning of the treatment (Table 1).
On days 7 and 14, a decrease in the number of granulocytes by 8 % and 14.8 % was noted, respectively in comparison to indicators of day 1, with a subsequent increase in their number by day 21. On day 28, an increase in the number of granulocytes was recorded by 0.6 % in comparison to day 1. By day 35, the number of granulocytes decreased again, remaining by 10.4 % lower than that before the beginning of the treatment.
Before the initiation of anticancer therapy, hematological signs of anemia were not observed in the studied animals. Fluctuations in hemoglobin level for the whole period of therapy did not exceed 2 %.
Prior to the beginning of therapy, an increase in the number of platelets by 16.5 % compared with the upper limit of normogram (117–460) was diagnosed. By the beginning of the second course of chemotherapy (on day 7), the platelet count was reduced by 9 % in comparison to day 1. In the following days, a gradual increase in the number of platelets was noted with its maximum by the 4th course of chemotherapy. On day 35, the platelet count exceeded the figures of the first day by 1.3 % (Table 1).
When assessing the overall toxicity on the WHO scale, no signs of toxicity higher than grade I in 6 animals were found (n = 6). Toxicity grade II manifested by a decrease in the appetite and activity was noted in one doggess (n = 1). Signs of development of stomatitis, gastrointestinal lesions, as well as other side effects, were not detected in all dogs. Patient 4, doggess, American bulldog breed, showed increased symptoms of eczema.
In order to determine the therapy effectiveness, an ultrasound determination of the size and structure of neoplasm was carried out before the treatment and on the 6th course of chemotherapy [2, 3]. For the study, one of the tumor nodes in each animal was selected (Figure 10).
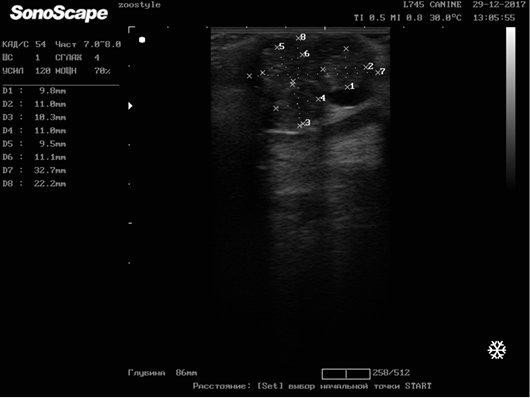
Figure 10: Longitudinal scanning of the mammary gland tumor (M5 region on the right side) on day 42 of the therapy (32.7х22.2 mm). Doggess, American Bulldog, at the age of 11.5 years.
The registered average degree of regression of a neoplasm was 14.02 % (± 1.69), which meets the criterion for the stabilization of the tumor process. Duration of the period of stabilization of tumor growth, since the last course of chemotherapy, was 103 (± 10.15) days (min–85 days, max–162 days).
CONCLUSION
The results of the studies convincingly show that the course of the combined systemic immune chemotherapy with Ligfol and Fluorouracil in doggesses with recurrent mammary gland cancer, according to the proposed scheme, has low overall and hematological toxicity that does not require delay in the treatment, dose reduction chemotherapy and corrective therapy. In addition, the proposed treatment strategy allows stabilizing the tumor process.
ETHICAL RESOLUTION
Ethical resolution was received from the commission of the Department of Morphology, Animal Pathology and Biology of the Federal State Budgetary Educational Institution of Higher Education Saratov State Agrarian University named after N.I. Vavilov of the Ministry of Agriculture of the Russian Federation.
SPONSORSHIP
Federal State Budgetary Educational Institution of Higher Education Saratov State Agrarian University named after N.I. Vavilov of the Ministry of Agriculture of the Russian Federation.
CONFLICT OF INTERESTS
Authors confirm the absence of conflict of interest in the article submitted for publication.
AUTHORS CONTRIBUTION
All authors contributed equally.
REFERENCES





