Advances in Animal and Veterinary Sciences
Research Article
Identification of Dermatomycetes Isolated from People and Animals with Dermatophytoses on the Territory of Kazakhstan
Yelena Kukhar*, Vladimir Kiyan, Ainur Smagulova, Anastassiya Nikulina
S. Seifullin Kazakh Agrotechnical University, 010011, Nur-Sultan, Prospect Pobedy, 62, Kazakhstan
Abstract | Dermatophytoses is a group of specific diseases caused by the dermatomycetes fungi. These diseases are common in humans and animals and are found ubiquitously. Recently, there have been increased numbers of fungal agents causing skin mycoses. This work aimed at the identification of dermatomycoses pathogens in humans and animals circulating in the territory of Kazakhstan. A total of 509 samples of pathological material from animals and human volunteers from various regions of Kazakhstan have been studied. As a result, 167 pure cultures of fungi were isolated, and their cultural and morphological characteristics were studied. A total of 80 strains of dermatomycetes, yeasts, and molds have been identified, including the genetic one. The most frequent were molds–64.3 %, dermatomycetes–22 %, and yeast–13.7 %. Changes had been noted in the range of dermatophytoses pathogens in humans and animals in the Republic of Kazakhstan. Pathogens of dermatophytoses in animals and humans were mold saprophytic fungi (64.3 %), dermatomycetes (22 %), and yeasts (13.7 %). The widest range of atypical pathogens was detected in the imported cattle.
Keywords | Dermatophytoses, Dermatomycetes, Identification, Microscopy, Pure culture, Typing, Microsporia, Trichophytia, Rubromycosis
Received | June 12, 2019; Accepted | August 30, 2019; Published | October 15, 2019
*Correspondence | Yelena Kukhar, S. Seifullin Kazakh Agrotechnical University, 010011, Nur-Sultan, Prospect Pobedy, 62, Kazakhstan; Email: kucharev@mail.ru
Citation | Kukhar Y, Kiyan V, Smagulova A, Nikulina A (2019). Identification of dermatomycetes isolated from people and animals with dermatophytoses on the territory of Kazakhstan. Adv. Anim. Vet. Sci. 7(s1): 21-27.
DOI | http://dx.doi.org/10.17582/journal.aavs/2019/7.s1.21.27
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Kukhar et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
In Kazakhstan, dermatophytoses of various etiology and localization are widespread among people and animals (Levchenko (2005), Bayduysenova (2007), Schurikhina (2010), Baydusenova et al., 2006; Schurikhin, 2009; Levchenko et al., 2005). Among people, rubromycoses, dermatophytoses, athlete’s feet of various etiology, onychomycoses, candidiases, as well as zoophilous microsporia and trichophytia have been identified. The most epidemiologically important are Trichophyton (T) verrucosum, T. mentagrophytes, T. rubrum, Microsporum canis. Among animals, T. verrucosum and M. canis have been identified most frequently (Kukhar et al., 2006).
Studies of the prevalence of the anthropophilic and zoophilous dermatophytoses, skin dermatomycoses, athlete’s feet and onychomycoses performed by various authors have shown that these diseases are very common in various countries and in specific population groups (Weitzman et al., 1998; Gudnadottir et al., 1999; Ellabib et al., 2002; Dogra et al., 2002; Ungpakorn et al., 2004; Djeridane et al., 2006; Borman, 2007; Ngwogu and Otokunefor, 2007; Anane et al., 2007; Sarma et al., 2008; Fekiha et al., 2012). Dermatomycoses and yeasts are the most frequent pathogens of skin mycoses in humans and in animals; however, the percentage of their identification varies depending on the geographical region. There are reports about changes in the spectrum of dermatophytoses pathogens (Gainullina and Panin, 2009).
The high frequency of occurrence and the wide range of pathogens require changes in the strategy and the tactics of diagnostic and therapeutic measures. Currently, microscopy and isolation of the pathogen cultures are proposed for diagnostics of dermatophytoses; however, intraspecies fungi identification using these methods is hindered. The share of the identified pathogens using the cultural method is rather low: 22–61.5 %, the average sensitivity of microscopy is 53.8 %. The laboratory diagnostics of mycoses is complicated by the fact that fungi structure varies considerably depending on the cultivation conditions. The reasons for untimely diagnosis are the incomplete examination of patients with the diseases, restricted to clinical examination and fluorescence examination; the frequency of atypical manifestations of fungal infections that has increased to 34 %; and the changes in the spectrum of pathogens (Novoselov et al., 2003).
This research work was aimed at identifying dermatomycetes isolated from patients and animals with dermatophytoses using classical and molecular genetic methods.
MATERIALS AND METHODS
Samples of pathological material from the animals were taken according to the requirements of veterinary clinics of Astana and livestock breeding farms in the Akmola, North Kazakhstan, and Karaganda regions. Human samples were taken from anonymous volunteers in outpatient hospitals, nursing homes, and the drug rehab clinic. It was obtained from volunteers agreed to fence biological material. The total of 509 samples of the pathological material were taken, of which 254 samples were taken from animals, and 255 samples were taken from humans.
For primary isolation of the fungi, Sabouraud’s slope agar was used. The clinical material had been soaked in 70 % alcohol for 20 minutes. The material was sown in three repetitions for each sample. Cultivation was performed on slope agar at pH of 6.5–6.9, and the temperature of 28º C for 5 to 30 days. The pure culture was isolated on agar in Petri dishes. For studying the morphological peculiarities of saprophytic fungi and dermatomycetes, agar blocks and Scotch preparations were prepared, and microscopy of smears in 50 % glycerol was made (Kurasova et al., 1971). The fungi were identified using appropriate identifiers (Sutton et al., 2001).
For genetic identification, multilocus sequence typing with primers ITS5 and ITS4, NS1 and NS4, RPB1 and RPB2, SSU4 and SSU5 was used. The nucleotide sequences were analyzed and combined into the common sequence in software SeqMan (DNA Star), identified in Gene Bank using the BLAST algorithm (Clayton et al., 1995).
RESULTS AND DISCUSSION
The dermatophytoses occurrence rate in Kazakhstan was analyzed according to the data of the Ministry of Health and the Ministry of Agriculture of the Republic of Kazakhstan. It was found that dermatophytoses in humans were quite common and were caused by various pathogens.
The trichophytia occurrence rate in the population of the Republic in 2000 was 4.7 per 100,000 of population, in 2007, the value increased to 9.68, and the peak in the Republic of Kazakhstan was observed in 2004–12.75. With that, the incidence rate of people with trichophytia in nine regions of the Republic over seven years was above the average (11.6): South Kazakhstan (34.9), Akmola (20.4), Aktobe (16.8), Kyzylorda (14.8), Almaty (12.9), East Kazakhstan (12.5), Atyrau (12.5), Karaganda (12.3), and Zhambyl (12.0). Of the total number of pathogens, most commonly found in humans was Trichophyton rubrum (20.4 % of the total number of cases). Onychomycoses caused by Candidae sp. made 12.6 %, by Epidermophyton sp.–9.4 %, and by T. mentagrophytes var. interdigitale –7.6 %. In 24.9 % of cases, the species of the pathogen was not veraciously identified, in 4.9 % of cases, the results were negative. In Russia, this indicator in the same years was 2.5, while in Uzbekistan it was 13.4 (Kukhar et al., 2012).
A tendency to increasing the incidence rate of microsporia has been identified from 2.574 thousand in 2001-8.153 thousand in 2002. Microsporia incidence rate currently remains quite high, although between 2002 and 2007, a clear tendency to reducing the incidence rate was observed. In 2007, the number of microsporia cases was 5.282 thousand (Levchenko, 2007).
Cattle trichophytia is identified throughout the entire territory of Kazakhstan. Cases of the disease were observed in the Akmola and the Karaganda regions. Mass skin lesions caused by pathogens of trichophytia were observed among animals in the imported meat livestock. With that, the disease often progresses in the malignant form, causing atrophy and defects of skins, has an atypical clinical picture, and may not always be treated with vaccine preparations. These lesions can be attributed to changes in the fodder base, the effects of stress during transportation and keeping at the quarantine site, prolonged adaptation to new territorial and climatic conditions, and by the absence of immunity to the disease, which results in the appearance of previously non-existent species of pathogens of skin lesions, as identified during the studies in a number of farms in the Akmola, Karaganda and North Kazakhstan regions (Kukhar et al., 2012).
In analyzing the primary sowings of the material (Table 1), growth of dermatomycetes–classic pathogens of dermatophytoses: Trichophyton sp. and Microsporum sp – was observed in 13.6 % of cases. In addition to the characteristic growth of dermatomycetes, the authors had identified typical growth of mold fungi Mucor sp., Penicillium sp., Aspergillus sp., Stemphyllium sp., Alternaria sp., Phoma sp., Chaetomium sp., Eurotium sp., and other soil fungi. Their share was 33.2 % of all the identified micromycetes. Growth of various yeasts was observed in 10.2 % of cases.
Table 1: Analysis of dermatophytoses pathogens isolation from the pathological material.
| Isolated fungi | Total samples – 509 | Total (%) | |||||
| Including from animals – 254 | Including from humans – 255 | ||||||
| Skin scrapes | Hair | Claws | Skin scrapes | Hair | Nails | ||
| Dermatomycetes | 9 | 12 | 3 | 2 | - | 43 | 69 (13.6) |
| Saprophytic fungi | 21 | 56 | 12 | 2 | - | 78 | 169 (33.2) |
| Yeasts | 27 | - | 2 | 2 | - | 21 | 52 (10.2) |
| Absence of growth | 24 | 67 | 21 | 5 | 1 | 101 | 219 (43.03) |
| Total: | 81 (15.9) | 135 (26.5) | 38 (7.5) | 11 (2.2) | 1 (0.2) | 243 (47.7) | 509 |
The yeasts were further identified as Candida sp. Rhodotorula sp., and Malassesia sp. Growth of micromycetes on nutrient media featured high speed, and their colonies were formed in 3–5 days. Growth of trichophyton colonies was observed on days 5–10, of microsporia–on days 3 – 6. Colonies of yeasts were formed on days 1–3. In 43 % of cases (219 samples), growth was not observed.
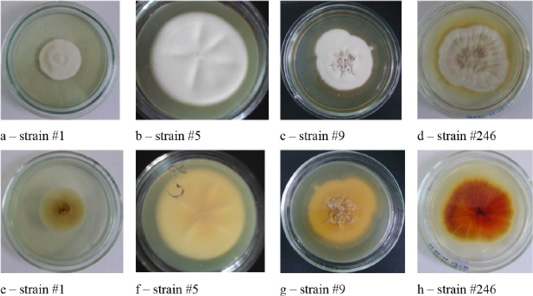
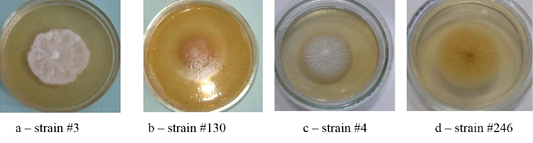
Figure 1: Colonies of isolates Trichophyton interdigitale. In the figure, letters a, b, c, and d show the front side, and letters e, f, g, and h show the reverse side of the colony.
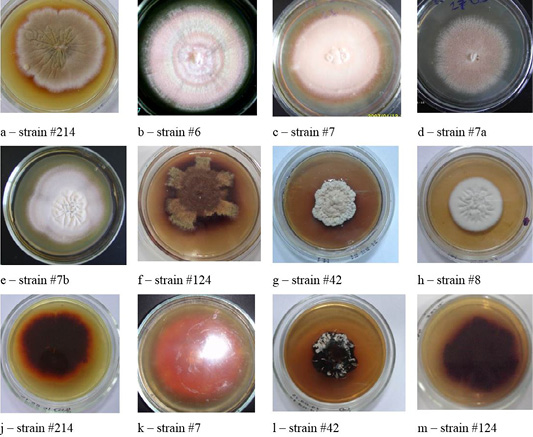
Figure 2: Colonies of clinical isolates of T. rubrum. Letters a, b, c, d, e, f, g, and h in the figure denote the front side, letters j, k, l, and m denote the reverse side of the colonies.
As shown by the data in Table 1, the highest percentage of isolation from the animal and human pathological material was observed for nondermatophyte fungi, including molds. The second occurrence rate was observed for dermatomycetes.
Pure cultures of dermatophytoses pathogens were identified by classical and molecular genetic methods.
The cultural properties of dermatomycetes colonies featured a variety of properties. On agar, there were isolated, often rounded, opaque colonies with abundant or scarce growth with a diameter of 3.0 – 7.0 cm. The edges of the colonies were often smooth, but there were also curl-shaped, indented, and wavy edges. The profile of the colonies was convex; the color was often white. The phenomena of polymorphism and pleomorphism were observed, especially in later reseeding (Figures 1, 2, 3 and 4).
Colonies of T. interdigitale were farinose, velvety or powdery, usually folded, all had solid consistency with a pronounced pigment of various shades of brown (Figure 1).
Colonies of T. rubrum featured many forms and colors. A characteristic feature was the accumulation of the wine-red pigment of varying intensity. The color of the colonies was white, beige, some strains were pink (Figure 2).
Colonies of T. verrucosum were leathery, velvety or powdery with moderate growth, regular rounded shape, of dense consistency, with the diameter of 30–50 mm, the edges were smooth, the color was white, the profile – convex, and the surface–wrinkled. The substrate mycelium grew to the inside and firmly connected with the dense nutrient medium (Figure 3).
The colonies of strains M. canis were floculent, creeping or dense powdery, of white to brown color. Colonies of T. tonsurans were creamy, friable, had a beige color with expressed pigment on the reverse side of the colony (Figure 4).
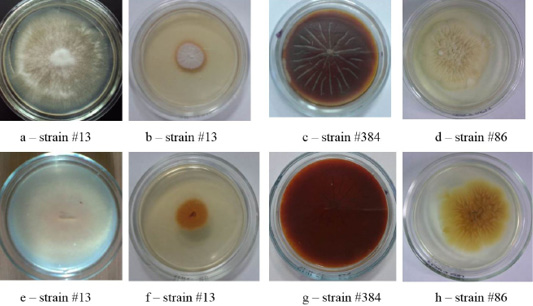
Figure 4: Clinical isolates of M. canis and T. tonsurans (strain #86). The Figure shows the front (a, b, c, d) and the reverse side of the colony (e, f, g, h).
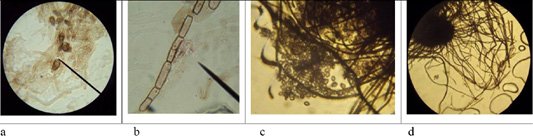
Figure 6: Morphological structures of microscopic fungi. Conidia (a) and mycelium (b) of micromycetes of Stemfyllium sp. #5.2; globular ascospores (c) and bristles (d) of Chaetomium globosum #129.
As one can see in Figures 1, 2, 3 and 4, the pathogen of rubromycosis T. rubrum features the characteristic pigment of intense wine-red color, the pathogen of plaster trichophytia T. interdigitale features the presence of a pigment of light-yellow color and dome-shaped elevation in the center of the colony, the pathogen of microsporia features pronounced polymorphism, characterized by a formation of intensive fluffy mycelium, dark brown color of the colonies, and mealiness.
Table 2: The results of the identification of micromycetes by using ITS and Sanger sequencing.
| # | Name and number of strains | Results of genetic identification of micromycetes |
| 1 |
No. 4 Trichophyton verrucosum |
Trichophyton verrucosum |
| 2 |
No. 5 Trichophyton mentagrophytes |
Trichophyton interdigitale |
| 3 |
No. 5.2 Stemfyllium sp. |
Stemphylium botryosum |
| 4 |
No. 6 Aspergillus sp. |
Aspergillus versicolor |
| 5 |
No. 6 Trichophyton verrucosum |
Trichophyton verrucosum |
| 6 |
No. 12 Chaetomium globosum |
Chaetomium globosum |
| 7 |
No. 13 Microsporum canis |
Microsporum canis |
| 8 |
No. 41 Trichophyton verrucosum |
Trichophyton verrucosum |
| 9 |
No. 86 Trichophyton tonsurans |
Trichophyton tonsurans |
| 10 |
No. 123.2 Ch. globosum |
Chaetomium nigricolor |
| 11 |
No. 128 Ch. globosum |
Chaetomium iranianum |
| 12 |
No. 129 Ch. globosum |
Chaetomium globosum |
| 13 |
No. 130 Trichophyton verrucosum |
Trichophyton verrucosum |
| 14 |
No. 144 Trichophyton rubrum |
Trichophyton rubrum |
| 15 |
No. 146 Trichophyton rubrum |
Trichophyton rubrum |
| 16 |
No. 152 Aspergillus sp. |
Eurotium rubrum |
| 17 |
No. 182 Alternaria alternata |
Alternaria alternata |
| 18 |
No. 208.2 Trichophyton mentagrophytes var. gypseum |
Trichophyton mentagrophytes var. gypseum |
| 19 |
No. 214 Trichophyton rubrum |
Trichophyton rubrum |
| 20 |
No. 302 Trichophyton verrucosum |
Trichophyton verrucosum |
| 21 |
No. 327 Trichophyton interdigitale |
Trichophyton interdigitale |
| 22 |
No. 627 Aphanocladium sp. |
Aphanocladium aranearum |
| 23 |
No. 1748 Penicillium sp. |
Penicillium dipodomyicola |
| 24 |
No. 16010 Arthroderma vanbreuseghemii |
Arthroderma vanbreuseghemii |
| 25 |
No. H11 Phoma macrostoma |
Phoma macrostoma |
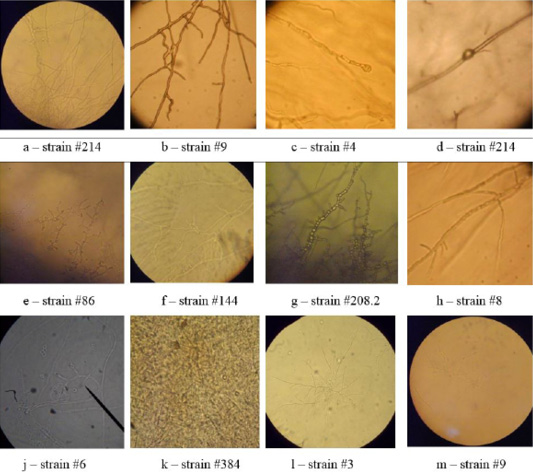
Microscopy of smears of dermatophytic fungi reveals clearly visible branching septate colorless mycelium, terminal and intercalary chlamydospores, micro and macroconidia (Figure 5).
On agar blocks, the start of forming growth tube was observed, which was accompanied by mycelium growth from the center, and in old cultures of dermatomycetes of genus Trichophyton, arthrospores and chlamydospores were found. Figure 6 shows septate branching mycelium T. verrucosum (a), coiled mycelium (b), terminal chlamydospores T. mentagrophytes (c), intercalary chlamydospores T. rubrum (d), microconidia T. tonsurans (e), microconidia T. rubrum (f), arthrospores T gypseum (g), T. interdigitale (h), micro- and microconidia T. verrucosum (j), microconidia M. canis (k), growth tubes of T. rubrum (l), and formation of a colony of T. verrucosum (m).
In the Gram-stained smears, the mycelium of dermatomycetes was presented in the form of soft thin septate filaments, and, when stained, had a light purple color. Microconidia had darker color and were tightly attached to the mycelium.
The microscopic analysis of mold fungi allowed identification by the presence of characteristic morphological features (Figure 6).
Next, genetic identification of fungi was performed using multilocus Sanger sequencing. The nucleotide sequence of target genes of dermatomycetes and molds was determined at the collective use laboratory of RSE “National Center for Biotechnology” of the Scientific Committee of the Ministry of Education and Science of the Republic of Kazakhstan (hereinafter refered to as the MES of the RK). After performing PCR on two pairs of primers ITS4-ITS5 and D1-D2, DNA samples were purified for sequencing. After decoding the DNA, the results were populated into the database on website www.ncbi.com with the use of the international BLAST database, and the obtained results were analyzed and compared to the data of the website. As a result, 80 strains of fungi were identified. The nucleotide sequences of the studied species were deposited in NCBI GenBank data base (Table 2).
CONCLUSION
The results of the research show a wide spread of dermatophytoses, which is also confirmed by statistical data of the MES of the RK. Along with the characteristic pathogens, which include dermatomycetes fungi, several uncharacteristic pathogens were identified, including Chaetomium globosum, Alternaria alternate, Aspergillus versicolor, and other saprophytes and yeasts. The results of the studies are consistent with the data presented in the works of the employees of the Reference Mycological Laboratory and other authors (Kurasova et al., 1971; Kukhar et al., 2012).
The combination of classical mycological and modern molecular-genetic methods made it possible to study the species diversity of pathogens of onychomycoses, trichophytia, and microsporia in the residents of Astana, in the animals in Astana, the Akmola, the North Kazakhstan, the Karaganda regions, and to identify them up to species with high accuracy. According to the results of studying pheno- and genotypic characteristics of the identified dermatomycetes, the main pathogens of rubromycosis, trichophytia, and microsporia in humans are mold saprophytic fungi (64.3 %) rather than classic dermatomycetes (22 %). Yeasts were isolated in 13.7 % of cases. The widest range of atypical pathogens was detected in the imported beef cattle (Sergeev, 2008). Therefore, in Kazakhstan, along with dermatomycetes, pathogens of dermatophytoses in humans and animals are yeasts, soil fungi, phytopathogens, and other micromycetes.
The comparison of the phenotypic and the genotypic characteristics has shown that the results of the studies are not 100 % similar. The results of the studies are consistent with the work of the scientists who reported insufficient correctness of primers ITS and SSU when working with dermatomycetes (Frealle et al., 2007).
Therefore, the following final conclusions can be proposed:
1. 509 samples of skin, nails, and hair pathological material were studied; 167 pure cultures of fungi were isolated, and their cultural and morphological characteristics were studied. 95 cultures were defined using typical methods.
2. Genetic definition of fungi was performed, which were pathogens of skin mycoses circulating in the area of Northern Kazakhstan and Western Siberia, 80 species of dermatomycetes, yeast and fungi were identified.
3. Changes had been noted in the range of dermatophytoses pathogens in humans and animals in the Republic of Kazakhstan. Pathogens of dermatophytoses in animals and humans were mold saprophytic fungi (64.3 %), dermatomycetes (22 %), and yeasts (13.7 %). The widest range of atypical pathogens was detected in the imported cattle.
ACKNOWLEDGMENTS
The article has been prepared based on the results of the research within the framework of a grant on topic “Phenotypic and Molecular Genetic Characteristic of Dermatomycoses Pathogens and Creating Test Systems for the Diagnostics of Microsporia, Rubromycosis and Gypsum Trichophytia” for 2012 – 2014 within program 055 “Scientific and/or Technical Activities” of the MES of the RK.
The authors are grateful to the employees of the collective use laboratory of the RSE “National Center for Biotechnology” of the MES of the RK, who participated in determining the nucleotide sequence of the target genes of dermatomycetes, and to the employees of the Research Institute of Agricultural Biotechnology, Laboratory of Fungi Biotechnology, and Department of Microbiology and Biotechnology at Saken Seifullin Kazakh Agrotechnical University (KATU), who have provided their assistance in the studies of cultural and morphological properties of fungi.
Conflict of interest
We declare that there are no conflict of interests.
AUTHORS CONTRIBUTION
All the authors contributed equally.
REFERENCES