Advances in Animal and Veterinary Sciences
Research Article
The Effects of Parity on Protective Protein Components and MMP Proteolytic Activity of Mammary Secretion during Involution in Tropical Dairy Cows
Attapol Tiantong*, Danford Augustino Mwabena
Faculty of Animal Sciences and Agricultural Technology, Silpakorn University, Phetchaburi IT Campus, Phetchaburi, 76120, Thailand.
Abstract | Several factors affect milk production in cows, including parity which can affect milk quality and health of cows. One of the essential process that occurs in the mammary gland at the drying stage is known as Involution which removes the milk-producing epithelial cells when they become redundant. In this stage, also, the γ-globulin (Ig), lactoferrin (Lf), and bovine serum albumin (BSA) levels which are the major protective proteins in the mammary secretion increased strongly. Matrix metalloproteinase-2 and -9 (MMP-2, -9) are enzymes secreted by leukocytes that play an important role in the remodeling mechanisms of the mammary gland during involution. Therefore, the objective of this study was to compare the protective protein components and the proteolytic capacity of the mammary secretion in primiparous and multiparous cows during the first 2 weeks after drying off. Six crossbred Holstein dairy cows with differential parity, including the 1st, 2nd, and 3rd parities, were used in this study. Mammary secretions were collected from the four quarters on d 0, 3, 7, and 14 of drying off and analyzed for protective protein components and the proteolytic capacity. Results showed that the total protein concentration and the proteinous component bands, including Ig, Lf, and BSA, were significantly higher in multiparous cows than in primiparous cows (P < 0.05) on d 7 and 14 of drying off. In contrast, the casein protein level decreased in multiparous cows compared to primiparous cows on d 7 and 14 of drying off. The level of MMP-9 in the mammary secretion of primiparous cows increased temporarily only on d 3, whereas that of multiparous cows gradually increased up to d 7. It can be concluded that the mammary glands in the multiparous cows underwent significant changes during the dry period. These changes most probably indicate the need of the mammary glands to deal with the characteristics of the involution.
Keywords | Parity, Proteolytic activity, Mammary secretion, Involution, Dairy cow
Received | March 30, 2019; Accepted | August 01, 2019; Published | September 25, 2019
*Correspondence | Attapol Tiantong, Faculty of Animal Sciences and Agricultural Technology, Silpakorn University, Phetchaburi IT Campus, Phetchaburi, 76120, Thailand; Email: tiantong_a@su.ac.th
Citation | Tiantong A, Mwabena DA (2019). The effects of parity on protective protein components and mmp proteolytic activity of mammary secretion during involution in tropical dairy cows. Adv. Anim. Vet. Sci. 7(10): 914-920.
DOI | http://dx.doi.org/10.17582/journal.aavs/2019/7.10.914.920
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Tiantong and Mwabena. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Since 1960, the dairy industry in Thailand had been developing till the milk production reached to 3400 tons per day or to more than one million tons per year as estimated in 2018, in which, this levels had been increasing linearly every year since 2015. About 40% of the milk production used by the school milk program and the remaining by the commercial dairy sector. Although Thailand is the largest producer and exporter of dairy products among the ASEAN countries, the milk production potential needs further improvement. The productive performance of smallholder dairy farm in the tropical countries is affected by various factors such as the breed, geographical location, season of calving, sucking status, body condition score, age, and parity (Obese et al., 1999; Masama et al., 2003; Msangi et al., 2005; Lobago et al., 2007). Yoon et al. (2004) reported that milk yield was lower in the first calving season than in other calving seasons and showed an increasing trend until the 5th parity and then decreased. They also found that milk composition tended to be reduced until the 5th parity and then recovered.
Dairy cow must be provided by a dry period for optimal milk production in the following lactation (Andersen et al., 2005). Although suppression of milk production is essential for proper cell renewal during early involution, the mammary gland is still susceptible to new intramammary infection (IMI) during this period (Smith et al., 1985; Leelahapongsathon et al., 2016). After the completion of early involution of the mammary gland, the risk of acquiring a new IMI is minimized (Lanctot et al., 2017). Oliver and Smith (1982) proposed that speeding up the recovery process of the mammary gland after the dry period could enhance its resistance to new IMI during early involution.
Polymorphonuclear neutrophils (PMNs) and macrophages represent the two major defense cell in the mammary gland (Paape et al., 2000; Peng et al., 2013; Tiantong et al., 2015a, b). PMNs count increased substantially during the early dry period (Chou et al., 2009; Ho et al., 2010; Yu et al., 2012). The matrix metalloproteinase (MMP) is a multifunctional protein that is secreted from the endothelial cells, PMNs, and macrophages and plays important roles in the degradation of the extracellular matrix (ECM), cell migration, cell apoptosis, and inflammation. A prominent accumulation of ECM-degrading MMP-2 and -9 has been reported in some studies during this early stage of involution (Miller et al., 2006; Chen et al., 2007; Weng et al., 2008; Yu et al., 2012). These modifications in the mammary gland reflect its physiological and immunological adaptations during the acute phase of involution.
The γ-globulin (Ig), bovine serum albumin (BSA), and lactoferrin (Lf) are the major proteinous components of the mammary secretion of dairy cows during the process of involution. Blood Ig is a polyreactive antibody that is spontaneously produced against environmental and self-antigens, which possesses potent opsonic and toxin-neutralizing properties (Hill, 1981; Baumgarth et al., 2005). Milk Ig is a primary exudate of blood and can protect the mammary gland against intramammary pathogens. BSA has the ability to bind, transport, and dispose the xenogenous compounds that gain entry into the animal body. The bacteriostatic function of Lf is primarily attributable to its iron-chelating property, and the N-terminal cationic peptide lactoferrin has the ability to disrupt the bacterial cell membrane. It also had immunomodulatory and anti-inflammatory functions by preventing bacterial lipopolysaccharides from initiating the host signal pathways (Legrand et al., 2004). Together, Ig, BSA, and Lf can provide immediate and broad protection to the mammary gland during the process of involution (Tiantong et al., 2015c).
This study was conducted to investigate the proteinous components and the proteolytic capacity of the mammary glands during the early phases of involution and to compare these parameters between primiparous and multiparous dairy cows.
METHODOLOGY
Animals and Management
Six crossbred Holstein cows with differential parity (1st, 2nd, and 3rd parities) were raised and mated at the dairy farm of Phetchaburi College of Agriculture and Technology (Phetchaburi, Thailand). Their milk yields were less than 5 kg/d, and the cows were more than 220 days in milk within the last 2 months of their 2nd, 3rd, or 4th gestation at the time of the current experiments. They were fed on a total mix ration (14% crude protein; 2500 kcal/kg metabolic energy) one week prior to milk stasis and had free access to rice straw and water. The sampling procedure and animal use protocols have been approved by the Committee on the Care and Use of Experimental Animals of Silpakorn University, Thailand.
Sampling and Sample Preparation
A 25-mL of mammary secretion was manually collected from each quarter at d (0) prior to dry-period and then d 3, 7, and 14. Mammary secretions from the four quarters were separated for each cow. All quarter secretion samples were transported in ice bath for preparation. It was skimmed by centrifuging at 400 × g for 20 min at 4°C (Tiantong et al., 2015a, b), and stored in aliquots at a temperature below −20°C for Bio-Rad protein assay, SDS-PAGE, and Gelatin Zymography which were performed within 2 months.
Total Protein Concentration
Total protein concentration of the skimmed supernatant of mammary secretions were determined by Bio-Rad protein assay using a dry-binding reagent (Bio-Rad Laboratories, Hercules, CA, USA) in a microplate format (SPECTROStar Nano, BMG Labtech GmbH, Ortenberg, Germany). This concentration were then used to calculate the volume necessary for electrophoresis analysis (SDS-PAGE) and Gelatin Zymography.
Electrophoresis Analysis (Sds-Page)
The soluble proteinous components of the quarter’s secretion was determined by SDS-PAGE. A 10% resolving gel with the Laemmli reducing buffer system were used (Laemmli, 1970). Equivalent of 10 μg protein determined from Bio-Rad protein assay for the skimmed quarters secretion supernatant were first mixed with a 2X Native Sample Buffer (161-0738, Bio-Rad Laboratories, Hercules, CA, USA) before performing SDS-PAGE. Then, the gels were stained for 30 min with 0.1% Coomassie Blue R-250 in 40% methanol, 10% glacial acetic acid, and 50% distilled water; then destained for 30 min twice in 30% methanol, 7.5% glacial acetic acid, and 62.5% distilled water; and preserved in distilled water for band visualization. The plausible proteinous components were identified on air-dried gels, and the putative band size was defined as 110–150 kDa for γ-globulin, 70–80 kDa for lactoferrin, 50–60 kDa for BSA, and 25–30 kDa for casein. The respective band images were captured using Epson Stylus TX130 (Seiko Epson Corporation, Nagano, Japan) and quantified using the ImageJ software (v.1.50; National Institutes of Health, Bethesda, MD, USA). The integrated band area was then adjusted for the amount of protein loaded, and the greatest band area per µg of loading protein was obtained. For normalization, the dry secretion samples collected on different days from the same cow were resolved on the same gel in parallel with the molecular weight standards (Precision Plus Protein Standards, Bio-Rad).
Gelatinolytic Capacity
Gelatin Zymography was a derivation of native SDS-PAGE in which gelatin was inoculated into the resolving gel as a substrate. In brief, an aliquot of the skimmed quarter secretion containing 20 μg protein was first mixed (1:1) with a sample buffer of pH 6.8 consisting of 62.5 mM Tris-HCl, 25% glycerol, 4% SDS, and 0.01% bromophenol blue (Bio-Rad). It was then subjected to 7.5% native SDS-PAGE (Minigel, Bio-Rad) containing 0.1% bovine gelatin (Sigma-Aldrich) in the resolving gel. Then, the gels were incubated in a renaturing solution of 2.5% (v/v) Triton X-100 at room temperature for 30 min, completely dripped with distilled H2O, and then incubated overnight at 37°C in 50 mM Tris-base buffer, pH 7.4, containing 200 mM NaCl, 0.02% Brij-35, and 5 mM CaCl2 for gelatinolysis. The next day, the gels were stained for 30 min with 0.5% Coomassie Blue R-250 (Sigma-Aldrich) in 40% methanol, 10% glacial acetic acid, and 50% distilled water; then destained for 30 min in 30% methanol, 7.5% glacial acetic acid, and 62.5% distilled water; followed by replacing the destaining solution and then destained for another 30 min; and finally preserved in distilled water. MMP-2 and -9 were identified as clear bands against the blue background based on the molecular size of the digested band after overnight gelatinolysis at 37°C. The level of gelatinase was expressed as the area of the band after scanning captured using Epson Stylus TX130 (Seiko Epson Corporation, Nagano, Japan) and integrated using the ImageJ software (v. 1.50; National Institutes of Health, Bethesda, MD, USA). Samples collected on different experimental days from the same cow were resolved on the same gel in parallel with the reference.
Statistical Analysis
The data of total protein concentration, proteinous protein contents, and the level of gelatinase activity evaluated among days in the same quarters were analyzed using an analysis of variance and Fisher’s least significant difference. Significant differences among treatments were analyzed by an independent t-test using the R version 3.5.1 software. All results were presented as mean + SE. Statistical significance was considered at P < 0.05.
RESULTS
Total Protein Concentration
Total protein concentrations were gradually increased until d 14 of drying off in the multiparous cows with a significant increment on d 7 and 14 than the primiparous cows. However, the total protein concentration did not change throughout the tested days in the primiparous cows (Figure 1).
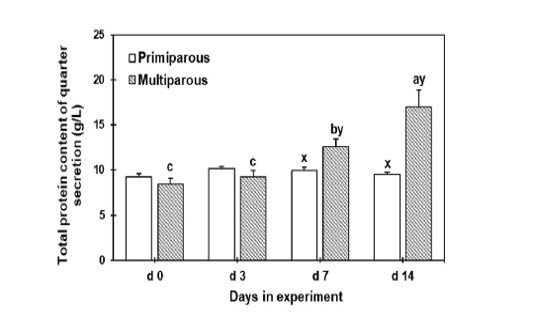
Figure 1: The total protein concentration of mammary secretion during the dry period for both primiparous and multiparous. a,b,c Columns carrying different superscripts are significantly different (P < 0.05) among different time points for the same treatment. x,y Columns carrying differences superscripts are significantly (P < 0.05) between treatment (primiparous and multiparous cows) at the same time point.
Protein Components of Secretions
Visually, the putative γ-globulin, lactoferrin, BSA, and casein bands of the mammary secretion are displayed in Figure 2. The levels of γ-globulin, lactoferrin, BSA, and casein (band area/µg protein) in the mammary secretion are shown in Figure 3.
γ-globulin and lactoferrin levels in the mammary secretion increased gradually (P < 0.05) from d 3 to 14 in the multiparous cows, with increment on d 7 and 14 than those of primiparous cows. The levels of BSA in the mammary secretion were increased (P < 0.05) on d 7 and remain high till d 14 in multiparous cows compared to those in primiparous cows. However, the level of casein in the mammary secretion were higher (P < 0.05) on d 0 and 3 in multiparous cows then decreased on d 7 and d 14 significantly (P < 0.05) than in primiparous cows. Whereas the primiparous cows showed no changes in the level of all proteinous components throughout the experiment.
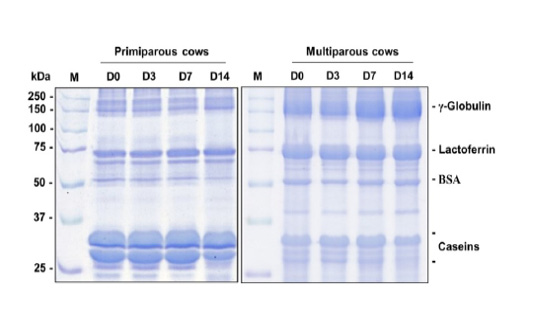
Figure 2: Images of γ-globulin, lactoferrin, BSA, and casein bands of the quarter secretion in 10% SDS-PAGE of primiparous cows (A) and multiparous cows (B) during the dry period.

Figure 3: The proteinous components levels of mammary secretion during the dry period for both primiparous and multiparous. a,b,c Columns carrying different superscripts are significantly different (P < 0.05) among different time points for the same treatment. x,y Columns carrying differences superscripts are significantly (P < 0.05) between treatment (primiparous and multiparous cows) at the same time point.
Mmp-2 and -9
Figure 4 displays, the gelatin zymogram of the mammary secretion supernatant. The progressive changes in the mean band areas of 92-kDa MMP-9 and 72-kDa MMP-2 are depicted in Figure 5. Both MMP-2 and -9 were detectable in the zymogram for both primiparous and multiparous cows. The MMP-9 levels in primiparous cows were significantly (P < 0.05) higher on d 3 than on d 0, 7, and 14, however, those of multiparous cows were significantly (P < 0.05) higher on d 7 and 14 than on d 0. Furthermore, the level of MMP-9 was significantly (P < 0.05) different between the primiparous and multiparous cows on d 0, 7, and 14 (Panel A, Figure 5).
The MMP-2 levels in both primiparous and multiparous cows were not significantly different among the various time points, however, the multiparous cows were significantly (P < 0.05) higher than those of primiparous cows throughout the experimental period (Panel B, Figure 5).

Figure 4: Images of MMP-2 and -9 bands of the quarter secretion in Gelatin Zymogram for both primiparous and multiparous cows.
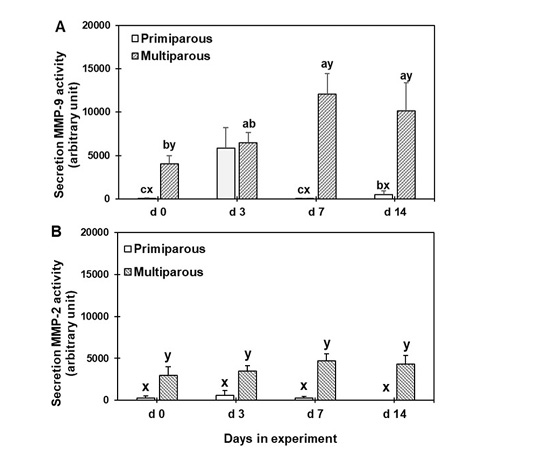
Figure 5: Gelatinolytic activity of mammary secretion during the dry period for both primiparous and multiparous cows. a,b,c Columns carrying different superscripts are significantly different (P < 0.05) among different time points for the same treatment. x,y Columns carrying differences superscripts are significantly (P < 0.05) between treatment (primiparous and multiparous cows) at the same time point
DISCUSSION
Some studies have reported that cow parity can had an effect on milk production and reproduction (Meikle et al., 2004; Yoon et al., 2004; Ángel et al., 2013), although, there is limited information about its effect on mammary gland remodeling during the acute phase of involution. Dry period of 45–60 days is believed to be indispensable in cows for optimal milk production in the subsequent lactation as it accelerates mammary secretory cell turnover (Weng et al., 2008). They also suggested that continuous milking in primiparous cows reduced subsequent milk yield by causing alterations in mammary epithelial cell turnover and secretory capacity. In this study, a progressive increase in the total protein concentration in the mammary secretion of multiparous cows was observed, which was similarly reported in Shamay et al. (2003). This increase can be attributed to a decreased gland activity in the dry period, with alterations in the mammary secretion composition caused due to decreased synthesis of casein, β-Lg, and α-La (Hurley and Rejman, 1986; Hurley, 1989; Aslam et al., 1994; Shamay et al., 2003) and also to changes in cell junctions and increased vascular permeability, thereby resulting in increased concentrations of blood serum proteins (Linzell and Peaker, 1971; Hurley, 1989; Boutinaud et al., 2003).
Immunoglobulin G1 (IgG1) is the most abundant Ig class in serum with a concentration of approximately 0.60 mg/mL, followed by sIgA 0.13 mg/mL, IgM 0.04 mg/mL, and IgG2 0.05 mg/mL (Butler, 1994). Milk IgG1 and IgG2 are derived from blood through FcRn-receptor mediated transcytosis (Baumrucker et al., 2010). However, milk IgA and IgM are locally produced and IgA release is accompanied by secretory component, or as sIgA (Ostensson and Lun, 2008). It was estimated that >90% of the γ-globulin in the mammary secretion compromised from both IgG1 and IgA.
The bacteriostatic and bactericidal activities of milk Lf could be less efficient during lactation because of its low concentration. However, during involution, milk yield would decrease dramatically, that could considerably augment the concentration of Lf (Rainard, 1983). This was confirmed in the results where the levels of γ-globulin, BSA, and Lf were gradually increased in the mammary secretion of multiparous cows.
Gelatinases are members of the MMP family, which are named after the zinc ion and the conserved methionine residue at the active site. MMP-9 represents the largest and the most complex member. Both MMP-2 and -9 have fibronectin type II which mediate binding to collagens and insertion into the catalytic domain (Chen et al., 2007; Page-McCaw et al., 2007).
Historically, gelatinases are believed to function primarily as enzymes that degrade the structural components of the ECM. In cows, the activity of these enzymes increases during involution (Tiantong et al., 2015b). The high gelatinolytic capacity of milk is a common phenomenon indicating the declining function of cow lactation to facilitate somatic cell transmigration and mammary tissue remodeling.
The ECM of the mammary gland is subject to dynamic remodeling throughout the lactation cycle (Capuco and Akers, 1999), and a slight alteration in its microenvironment which exerts significant impacts on the survival and function of mammary epithelial cells (Akers, 2002). The capacity of gelatinases of the mammary secretion reflects the remodeling of the mammary glandular structure under either spontaneous gradual or stasis-induced acute involution. The obtained results revealed that, the levels of the indicators (MMP-2 and -9) suggest that the mammary gland exhibited more ECM degradation activity during the acute phase of involution in multiparous cows than that in primiparous cows.
CONCLUSION
It can be concluded that the major protective proteins (γ-globulin, BSA, and lactoferrin) and proteolytic capacity (MMP-2 and -9) of the mammary secretion is associated with actual mammary tissue remodeling during involution in multiparous cows that indicate efficient cell renewal and modeling of the mammary gland required for the preparation of the next lactation.
ACKNOWLEDGMENTS
The authors are thankful to Silpakorn University for funding this study and the Phetchaburi College of Agriculture and Technology for providing the experimental dairy farm and helping collect the milk samples.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHORS’ CONTRIBUTION
The authors contributed equally.
REFERENCES





