Advances in Animal and Veterinary Sciences
Research Article
Effect of Peppermint and Rosemary Extractions on Ruminant In-vitro Digestibility
Jamal A. Tawfeeq1*, Hussein A. Al-Omrani2, Rasha M. Shaker3, Zaid R. Hamza1, Sarah F. Abbas1, Raed H. Jabbar1
1Department of Animal Production, College of Agriculture Engineering Science, University of Baghdad, Baghdad, Iraq; 2Department of Horticulture, College of Agriculture Engineering Science, University of Baghdad, Baghdad, Iraq; 3Biochemistry Section, College of Dentist, University of Baghdad, Baghdad, Iraq.
Abstract | The objective of this study was to evaluate the effects of organic, watery and alcoholic extractions of peppermint and rosemary leaves on in-vitro ruminant digestibility. Three tubes for each treatment with three blank were used and the mean value was evaluated statistically. It was found that after 48 hours of incubations, addition of 200mg of organic extractions for peppermint and rosemary significantly decreased dry matter digestibility (DMD) in contrast with control, by 83.41%, 81.99% and 86.21% respectively, and organic matter digestibility (OMD), 85.23%, 84.27% and 90.19% respectively, while there were no significances for adding 200mg of dried leaves, watery and alcoholic extractions. Results suggested that the addition of peppermint or rosemary as a dried herbage or fresh to ruminants’ diet didn’t alter the rumen digestibility with a high content of crude protein.
Keywords | Peppermint, Rosemary, In-vitro, Digestibility, Ruminants
Received | June 01, 2019; Accepted | July 24, 2019; Published | September 25, 2019
*Correspondence | Jamal A Tawfeeq, Department of Animal Production, College of Agriculture Engineering Science, University of Baghdad, Baghdad, Iraq; Email: drjamalani@yahoo.com
Citation | Tawfeeq JA, Al-Omrani HA, Shaker RM, Hamza ZR, Abbas SF, Jabbar RH (2019). Effect of peppermint and rosemary extractions on ruminant in-vitro digestibility. Adv. Anim. Vet. Sci. 7(10): 910-913.
DOI | http://dx.doi.org/10.17582/journal.aavs/2019/7.10.910.913
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Tawfeeq et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Peppermint, Mentha piperita or Mentha balsamea wild, and rosemary, Rosmarinus officinalis, are evergreen perennial shrub, were widely used in medicinal products, with large medical bioactive compounds, like menthol, menthone, isomenthone, eriocitrin, phenolic compounds (Sokovic et al., 2009; Hussain et al., 2010). In addition, leaves and oil of mint family used in food as flavorings and food seasoning, to contain volatile aromatic oils. These plants with fibrous roots are cultivated in many drought areas with lack of water for long periods, so, it’s important plants in subtropical climate. Civilizations of Mesopotamia and Nile valley were the first of using in medicine. These phytogenic additives and phytochemicals contain camphor, salicylate, kaifik, yurosolic and butylonic acid, antioxidants such as karnosic acid, rosmarinic acid and carnosol (Hassan et al., 2014). During grazing, animals’ ad-
libitum feeding peppermint and rosemary or others medical plants, there for, ruminant exposed to many phenolic compounds, like tannins, lignin and other metabolites like saponins. These aromatic plants have essential oils (derived components) which aren’t poison (FDA, 2004), active against many gram-positive or negative bacteria by interfering with permeability of cell membrane causing intracellular leakage (Trombetta et al., 2005), in order to the possibility of adaptation of rumen microflora, bioactive compounds in peppermint or rosemary suppressed the growth human viruses, so, it may have negative effect against rumen microorganisms. Thus, the aim of the following research was to determine the impact of peppermint and rosemary dried leaves, watery, alcoholic and organic extractions in rumen digestibility.
Table 1: Chemical composition of peppermint, rosemary and alfalfa hay (% as DM basis)
| Ash% | OM% | CF% | CP% | EE% | NFE% | pH | Phenolics (mg/ gm) | |
| Peppermint | 13.70 | 86.30 | 16.94 | 11.79 | 2.21 | 55.36 | 6.42 | 18.49 |
| Rosemary | 29.25 | 70.75 | 16.86 | 8.40 | 3.46 | 42.03 | 6.38 | 22.35 |
| Alfalfa hay | 7.99 | 92.01 | 19.86 | 19.36 | 2.11 | 50.68 | 6.76 | 5.87 |
Material and Methods
Preparation of Plant Extracts
The leaves and flowering plants of peppermint and rosemary were sampled and dried at 65°C in a ventilated oven for 48 -96h. then grounded at sieving 1mm. (Tawfeeq and Hassan, 2014), stems and woody parts were separated. Three methods of extractions, watery, alcoholic and organic were used at room temperature (Shtayeh and Abu Ghadeib, 1999; Hassan et al., 2014). Watery extracts, 50gm of samples were extracted in a 500ml beaker, fill it with boiled distill water with stirrer for 30 minutes then filtered with cheese cloth and dried at 40°C. Alcoholic extracts: 100g of peppermint or rosemary was extracted with 500 ml of 70% ethanol in a 1000ml beaker with stirrer for 24h., then filtered with cheese cloth, toke the supernatant fluid and dried at 40°C to evaporate alcohol and dried. Organic extract: 100gm of samples were weighted in a 1000ml beaker, fill it with hexane and soak for 24h. then extracted with a Soxhlet apparatus during 2h. All extracts were stored in deep freeze until use. Nutritional value was analyzed according to AOAC (2012) (Table 1).
Determination of Total Phenolic Content
Total phenolic were determined with Folin-Ciocalteu reagent. Gallic acid was used as a standard (we can use tannic acid, lignin or gallic acid), and the total phenolic were expressed photometrically as gallic acid equivalents to 100gm of raw material using Lambert-bear law “A = As×B×C” Where: A(absorption), As (0.966) from the calibration curve, B=1 (cell diameter) and the C (concentration of phenolic compounds (mg/ gm)). The Folin-Ciocalteu reagent reducing polyphenols and producing blue color which measured spectrophotometrically at 765nm (Cuvelier et al., 1996).
In-vitro Digestibility
In-vitro digestibility was implemented as Tilley and Terry (1963): Active sample of rumen liquor was collected from slaughtered sheep and incubated in water bath at 38°C, artificial saliva prepared by two solutions, first: McDougall’s Buffer solution, 49gm NaHCO3 + 18.6gm Na2HPO4 dissolve in 800ml DW, second: Chlorides solution, 23.5gm NaCl + 6gm MgCl2.7H2O + 28.5gm KCl + 2gm CaCl2 dissolved in 1000ml DW. Add 100ml from the second solution to the first one (800ml) and completed up to 1000ml then incubated in 38°c water bath. Digestion tubes with 0.5gm grinded alfalfa hay through a 1mm sieve were prepared with two levels of peppermint or rosemary extracts (0, 200mg per tube). A mixture of 40ml artificial saliva and 10ml rumen liquor was added to each tube then incubated for 48h in shaker water bath at 38°c with gentle shaking two times a day. After finished 48h centrifuged the tubes and dry the residue (precipitate) 105°c overnight, weight then ash at 600°c for 5 h. calculate the dry matter and organic matter digestibility. All chemical compositions were determined according to AOAC (2012) for dry matter (DM), wet, organic matter (OM), crude protein (CP), ether extract (EE), crude fiber (CF), ash and nitrogen free extract (NFE) as % of DM basis.
Statistical Analysis
One-way ANOVA analysis was performed to determine significant differences (P<0.05) between treatments, which was implemented using statistical program (SAS, 2012) and Duncan test (Duncan, 1955). Yij =μ+ti +δej
RESULTS and DISCUSSION
Effect of Peppermint or Rosemary on In-vitro Dry Matter Digestibility
Results of alfalfa dry matter digestibility (DMD) indicated to significant decreases (P<0.05) in digestibility with 200mg organic extraction of peppermint and rosemary in contrast with control, 83.41%, 81.99% and 86.21% respectively, while, no significance for dried leaves, watery and alcoholic extractions (Table 2), main effect of added extractions didn’t differ significantly in contrast with control (Figure 1), hexane is an organic solvent that is very efficient in dissolving fat and fat-like substances (such as vitamins and active soluble compounds), therefore, there is a possibility of increasing their concentration and affect negatively on in-vitro digestibility, the same changes of phytofactors availability to the microorganisms has been described by Castro-Montoya et al. (2011), Agarwal et al. (2009) observed decreased of in vitro digestibility of feed by inclusion peppermint oil in buffalo rumen fluid. The results of alcoholic and watery extractions or dried leaves agree with Zmora et al. (2012), they refereed to no effect on in-vitro dry matter digestibility when added dried leaves of menthe peppermint at levels 0, 2.33, 8.17, 16.34 and 23.35 mg for each tube, Azeez and Tawfeeq (2019) referred to no effects of eichhornia-crassipes on ruminant as roughage feed due to adaptation of ruminant to the presence of possible feeds around, for this reason, further efforts will be required to identify effective compounds able to positively affect rumen ecosystem, especially with in-vivo digestibility and continues flow rate of nutrients, water.
Table 2: Effect of peppermint and rosemary extractions on dry matter digestibility of alfalfa hay (%) ±SD
| Treatments | Peppermint | Rosemary |
| T1 |
86.21a ± 0.09 |
86.21a ± 0.09 |
| T2 |
88.33a ± 0.09 |
89.52a ± 0.09 |
| T3 |
85.89 a ± 0.32 |
85.99a ± 0.58 |
| T4 |
87.03a ± 0.87 |
84.09ab ± 0.67 |
| T5 |
83.41b ± 0.59 |
81.99b ± 0.25 |
Different letters in the same column indicate differences at 0.05 significance level, T1= alfalfa ( control), T2= alfalfa with 200mg of dried leaves, T3= alfalfa with 200mg watery extraction, T4 = alfalfa with 200mg alcoholic extraction, T5 = alfalfa with 200mg organic extraction.
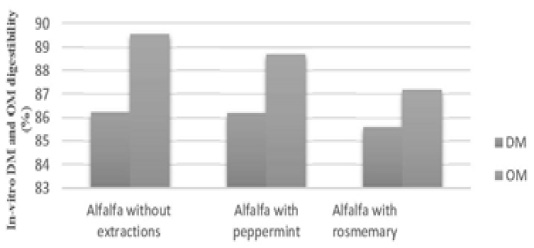
Figure 1: Main effects of in-vitro dry matter and organic matter digestibilty for alfalfa with or without extractions
Effect of Peppermint or Rosemary on In-Vitro Organic Matter Digestibility
Results of organic matter digestibility (OMD) indicated to significant decreases (P<0.05) for in vitro digestibility with 200mg organic extraction of peppermint and rosemary in contrast with control, 85.23%, 84.27% and 90.19% respectively, but no statistical difference was observed between dried leaves, watery and alcoholic extractions (Table 3). Digestibility affects by organic matters content, incubation time, proportions of rumen microorganisms, the relatively higher OM degradability may be according to the low content of ash (Table 2) or due to the phytofactors in extracts which it non degraded by rumen microorganisms. On the other hand, no differences for the main effects of added extracts in contrasted with OM digestibility of alfalfa hay (Figure 1), Hosoda et al. (2006) showed a limited effect of peppermint on rumen digestibility in dairy cows, while Cobellis et al. (2016) demonstrated that rosemary leaves may be used to modulate rumen microbiome and its function to more utilize rumen nitrogen. Some of active component as short chain fatty acids work as antioxidant and have more than one mode of action, so, the strong antioxidant activity my appeared with organic extractions. Secondary metabolites in plant extracts contain antimicrobial properties, but mode of action differs in extract rather than in plant, that may play the main role of non-significances for adding extract in contrasted with control (Figure 1) and increased apparent OM digestibility when fed 200mg/sheep/ day (Sahraei et al., 2014).
Table 3: Effect of peppermint and rosemary extractions on organic matter digestibility of alfalfa hay (%) ±SD
| Treatments | Peppermint | Rosemary |
|
T1 |
90.19a ± 0.09 |
90.19a ± 0.09 |
| T2 |
89.01a ± 0.13 |
88.11a ± 0.72 |
| T3 |
90.33 a ± 0.33 |
87.91a ± 0.43 |
| T4 |
88.90a ± 0.02 |
85.33ab ± 0.50 |
| T5 |
85.23b ± 0.05 |
84.27b ± 1.22 |
Different letters in the same column indicate differences at 0.05 significance level, T1= alfalfa ( control), T2= alfalfa with 200mg of dried leaves, T3= alfalfa with 200mg watery extraction, T4 = alfalfa with 200mg alcoholic extraction, T5 = alfalfa with 200mg organic extraction.
Acknowledgements
This study was supported by College of Agricultural Engineering Sciences, University of Baghdad. The authors are grateful to the dean of college and the head of Animal Production Department in addition to the head of Horticulture Department for their assistance during the experimental period.
CONFLICTS OF INTEREST
Authors declare that there are no conflicts of interest.
authors contribution
All authors contributed to the work, discussed the results and contributed to the final manuscript.
References





