Advances in Animal and Veterinary Sciences
Fermented Juice of Epiphytic Lactic Acid Bacteria and Molasses Addition on the Fermentation Characteristics and Nutrient Compositions of Sorghum Silage
Achara Lukkananukool1, Kanokrat Srikijkasemwat1, Amphai Promnaret2, Min Aung3, Yin Yin Kyawt3*
1Department of Animal Production Technology and Fisheries, Faculty of Agricultural Technology, King Mongkut’s Institute of Technology Ladkrabang, Bangkok, 10520, Thailand; 2National Corn and Sorghum Research Center, Faculty of Agriculture, Kasetsart University, Nakhon Ratchasima, 30320, Thailand; 3Department of Animal Nutrition, University of Veterinary Science, Yezin, Nay Pyi Taw, 15013, Myanmar.
Abstract | This study was conducted to determine the effect of fermented juice of epiphytic lactic acid bacteria (FJLB) and molasses addition on the fermentation characteristics and nutrient compositions of sorghum silage. Sorghums were chopped into pieces of 1-2 cm length and molasses 5% (w/w) and FJLB 1% (v/w) of fresh material were added as silage additives. No significant effects on physical characteristics except pH, lowest in molasses silage, were noted. The higher organic matter and lower fibre contents were observed in molasses silage. Total carbohydrate, non fibre carbohydrate, total digestible nutrients, digestible dry matter and dry matter intake were highest in molasses silage. The higher acetic, propionic and lactic acid concentrations were observed in FJLB silage. The lactic acid bacteria (LAB) count was highest in FJLB silage, and lowest in control silage. The low volatile based nitrogen (VBN) and high V-score, indicators of good quality silages, were generally observed in molasses and FJLB rather than control silages. This experiment indicated that FJLB can be alternatively used as additive instead of using molasses in sorghum silage; however more inclusion level of molasses and FJLB should be used to ensure good quality silage.
Keywords | Molasses, FJLB, Sorghum, Silage, V-score
Received | March 31, 2018; Accepted | May 01, 2019; Published | June 30, 2019
*Correspondence | Yin Yin Kyawt, Department of Animal Nutrition, University of Veterinary Science, Yezin, Nay Pyi Taw, 15013, Myanmar; Email: dr.yinyinkyawt81@gmail.com
Citation | Lukkananukool A, Srikijkasemwat K, Promnaret A, Aung M, Kyawt YY (2019). Fermented juice of epiphytic lactic acid bacteria and molasses addition on the fermentation characteristics and nutrient compositions of sorghum silage. Adv. Anim. Vet. Sci. 7(8): 668-673.
DOI | http://dx.doi.org/10.17582/journal.aavs/2019/7.8.668.673
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Lukkananukool et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Sorghum is becoming important forage especially in tropical regions because of its adaptability to environments with limited rainfall, high temperature and low soil fertility, and acceptable energy values and relatively high apparent dry matter degradability (>600g/kg DM) (Carbal Filho et al., 2005). Considerations with forage shortage during summer time, forage were conserved through the fermentation process of ensiling. However, the difficulty in ensiling of tropical forages is low concentration of WSC (McDonald et al., 1991), which resulted in lower fermentation quality, intake, and digestibility. Thus, the additives such as fermentation stimulants, fermentation inhibitors, aerobic deterioration inhibitors, nutrients and absorbents should be used to obtain the good quality silage.
Whole-crop cereal silages, such as corn and sorghum, are susceptible to aerobic deterioration, especially in warm climates (Filya, 2003). This is due to aerobic yeasts which are most active at 20-30˚C (Ashbell et al., 2002). Therefore, it is very important to find suitable additives that inhibit fungi and protect the silage against aerobic exposure. Among the several silage additives, molasses is the well-known additive for ensiling, and the fermented juice of epiphytic lactic acid bacteria (FJLB) is also the technique which has the ability to increase the silage quality of tropical forages. Lukkananukool et al. (2018) stated that FJLB can be alternatively used as additive instead of using molasses in cassava leaves silage.
The silage quality of sorghum forage and sorghum plus soybean improved with the addition of molasses and bacterial inoculant, respectively (Mahala and Khalifa, 2007; Lima et al., 2010). Thus, the quality of sorghum silages could be promoted with not only molasses but also bacteria inoculation; however, there are limited reports about the addition of FJLB to sorghum for silage making. Thus, this study was conducted to determine the effect of FJLB and molasses addition on the fermentation characteristics and nutrient compositions of sorghum silage.
Materials and methods
Preparation of FJLB
The FJLB from Napier Pak Chong1 grass was prepared 2 days prior to silage making. Each 200 g of fresh grass was macerated with 600 ml of distilled water using a blender. The macerate was then filtered through a sterilized double layer of cheesecloth and the filtrate was put into 600 ml flask. About 1% (w/v) of glucose was added into the filtrates and there were shaken well and kept in an incubator at 30°C for 2 days (Bureenok et al., 2005).
Silage Making
Whole plant sorghum were harvested in the field (Pak Chong, Nakhon Ratchasima Province, Thailand) and chopped into pieces of 1-2 cm length before ensiling. The molasses 5% (w/w) and FJLB 1% (v/w) of fresh material were added as silage additives and control silage was added with an equivalent amount of distilled water. Approximately 100 g fresh matter of treated crop then was packed into a plastic pouch in triplicate, and sealed with vacuum sealer (SQ202, Sharp, Co. Ltd, Japan). The bags were stored at room temperature and samples were taken at 21 days after ensiling for chemical analysis.
Measurements of Silage Quality
The physical characteristics of silages such as smell, taste, texture and color were assessed. For the determination of nutritional parameters, 20 g of fresh matter were extracted with 70 ml of distilled water and stored at 4°C for overnight. Then the extracts were filtered with filter paper and pH, ammonia nitrogen (NH3-N) and organic acid concentrations and V-score were measured. The pH of silage was measured by using a pH meter (F-23; Horiba, Tokyo, Japan). Volatile based nitrogen (VBN) and total nitrogen (TN) content were analyzed by using a steam distillation technique (Foss 2020 digester and Foss 2100 Kjeltec distillation unit, FOSS Analytical, Denmark) reported by Japan Grassland Farming Forage Seed Association (JGFFSA, 1994). Organic acid concentrations were determined by using High Performance Liquid Chromatography (Shim-pack SCR-102H, 300 mm × 8.0 mm id; column temperature, 40°C; flow rate, 0.8 mL/min, Shimadzu, Kyoto, Japan). The V-score was determined by method reported by JGFFSA (1994). Detail methods v score
Chemical Analysis
The rest silage materials are placed in the forced-air oven at 70˚C for 48 h to obtain the constant weight. After 48 h, all samples are immediately weighted and recorded it. All samples are ground with 1 mm sieve and analyzed for dry matter (DM), organic matter (OM), ether extract (EE) by the method described by AOAC (1990). Neutral detergent fibre (NDF) and acid detergent fibre (ADF) were analyzed according to the method of Goering and Van Soest (1970). All feeds were analyzed for nitrogen (N) by using Kjeldahl method (Foss 2020 digester and Foss 2100 Kjeltec distillation unit, FOSS Analytical, Denmark) and crude protein (CP) was calculated as 6.25 × N (AOAC, 1990).
Estimation of Nutritional Parameters of Silages
The nutritional parameters such as total carbohydrate (TC), non fibre carbohydrate (NFC), total digestible nitrogen (TDN), digestible dry matter (DDM) and dry matter intake (DMI) were estimated by the formulas, which are expressed as followed.
TC = 100-(CP+EE+ASH) (NRC, 2001)
NFC = 100-(NDF+CP+EE+ASH) (NRC, 2001)
TDN (%DM) =87.84-(0.70×ADF)
DDM (% DM) = 88.9-(0.779×ADF)
DMI (% of BW) =120/NDF (NRC, 2001)
Isolation of Lactic Acid Producing Bacteria
MRS (de Man, Rogosa and Sharpe) agar plates are used for the isolation of LAB. To distinguish acid-producing bacteria from other bacteria, 1% CaCO3, is added to the MRS-agar plates. Samples are incubated under anaerobic conditions at 30˚C for 3 to 5 days. Colonies of acid-producing bacteria, identified by a clear zone around each colony, are randomly selected from MRS-agar plates and purified by replacing on MRS-agar plates. Colonies were counted as viable numbers of microorganisms and are expressed as colony-forming unit per ml (log10 cfu/ml) (Kozaki et al., 1992).
Statistical Analysis
The data were subjected to the analysis of variance (ANOVA) and the significance of differences between means was compared by Duncan’s Multiple Range Test (DMRT) (Steel and Torrie, 1980) using SPSS (version 16.0) software.
Results
The physical characteristics of sorghum silages were presented in Table 1, in which no remarkably differences except pH were noted. The yellowish green color, firm soft texture, vinegar smell and acidic taste were generally observed in all silages. The pH values of experimental silages were significantly different (p<0.05) each other, wherein molasses silage had lowest (p<0.05) pH value, followed by FJLB and control silages.
Table 1: Physical characteristics of sorghum ensiled with different additives
| Descriptions | Treatment | ||
| Control | FJLB | Molasses | |
| pH |
5.31a |
5.00b |
4.49c |
| Color | Yellowish green | Yellowish green | Yellowish green |
| Texture | Firm soft | Firm soft | Firm soft |
| Smell | Vinegar | Vinegar | Vinegar |
| Taste | Acidic | Acidic | Acidic |
FJLB: fermented juice of epiphytic lactic acid bacteria
The significant variations (p<0.05) in chemical composition except DM were observed in Table 2. The OM content of control silage was significantly lower (p<0.05) than that of FJLB and molasses silage. As the CP content, molasses silage was not significantly different (p>0.05) with control and FJLB silages, whereas FJLB silage was lower (p<0.05) than control silage. The fibre (NDF and ADF) and EE contents of control and FJLB silage additives were higher (p<0.05) than those of molasses silage.
The nutritive values of experimental silages were shown in Table 3. The TC contents of FJLB and molasses silage additives were significantly higher (p<0.05) than that of control silage. The NFC, TDN, DDM and DMI of molasses silage were significantly greater (p<0.05) than those of control and FJLB silages, wherein the higher (p<0.05) TDN content and lower (p<0.05) DDM were observed in FJLB silage compared with control silage.
The organic acid concentrations except butyric acid were significantly different (p<0.05), which are expressed in Table 4. The acetic, propionic and lactic acid concentrations of FJLB silage was significantly higher (p<0.05) than those of control and molasses silages, which are not different (p>0.05) each other.
The LAB count, VBN and V-score of experimental silages were shown in Figure 1A, 1B and 1C, respectively. The LAB count of molasses silage additive was not significantly different (p>0.05) with control and FJLB silage additives, in which FJLB silage additive was significantly higher (p<0.05) than control. The VBN of FJLB silage additive was not different (p>0.05) with control and molasses silage additives, whereas lowest value (p<0.05) was observed in molasses silage additive comparison with control. The V-score of experimental silages were not statistically different (p>0.05) each other, however the highest value was observed in molasses silage additive, followed by FJLB and control.
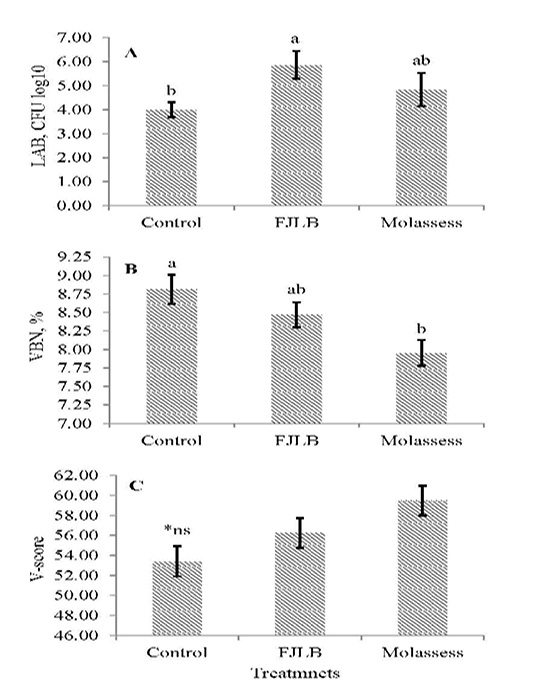
Figure 1: Lactic acid bacteria count (1A), volatile based nitrogen (1B), and V-score (1C) of sorghum ensiled with different additives
Discussion
The noteworthy dissimilarities on physical characteristics except pH were not observed in this experiment. The lowest pH value was observed in molasses silage additive, which was within the acceptable range for good quality silage in the tropics (Bilal, 2009; Nhan et al., 2009). Molasses are rich in readily fermentable WSC and stimulates fermentation process, thereby reducing pH of silage (McDonald et al., 1991; Yunus et al., 2000; van Neikerk et al., 2007). Mahala and Khalifa (2007) and Lima et al. (2010) showed that molasses addition decreased pH content of sorghum and sorghum plus soybean silages, respectively. Addition
Table 2: Chemical compositions of sorghumensiled with different additives
| Descriptions | Treatment (Means) | SEM | P value | ||
| Control | FJLB | Molasses | |||
| DM, % | 33.96 | 34.11 | 35.69 | 0.66 | 0.566 |
| OM, % DM |
92.87b |
93.09a |
93.19a |
0.05 | 0.006 |
| CP, % DM |
7.34a |
6.80b |
7.12ab |
0.10 | 0.064 |
| NDF, % DM |
53.44a |
55.09a |
44.84b |
1.61 | <0.001 |
| ADF, % DM |
36.58b |
39.98a |
30.29c |
1.44 | <0.001 |
| EE, % DM |
1.72a |
1.39b |
1.01c |
0.11 |
0.001 |
DM: dry matter, OM: organic matter, CP: crude protein, NDF: neutral detergent fibre, ADF: acid detergent fibre, EE: ether extract, FJLB: fermented juice of epiphytic lactic acid bacteria, SEM: standard error mean
All chemical compositions except DM are dry matter basis.
Different superscripts in the same row are significantly different at P <0.05.
Table 3: Nutritive values of sorghum ensiled with different additives
| Descriptions | Treatment (Means) | SEM | P value | ||
| Control | FJLB | Molasses | |||
| TC, % DM |
83.81b |
84.90a |
85.06a |
0.22 | 0.008 |
| NFC, % DM |
30.37b |
29.81b |
40.22a |
1.70 | <0.001 |
| TDN, % DM |
59.85c |
62.24b |
66.64a |
1.01 | <0.001 |
| DDM, % DM |
60.41b |
57.75c |
65.31a |
1.12 | <0.001 |
| DMI, % BW |
2.25b |
2.18b |
2.68a |
0.08 | <0.001 |
TC: total carbohydrate, NFC: non fibre carbohydrate, TDN: total digestible nitrogen, DDM: digestible dry matter, DMI: dry matter intake, FJLB: fermented juice of epiphytic lactic acid bacteria, SEM: standard error mean
Different superscripts in the same row are significantly different at P < 0.05.
Table 4: Organic acid concentrations of sorghumensiled with different additives
| Descriptions | Treatment (Means) | SEM | P value | ||
| Control | FJLB | Molasses | |||
| Acetic/A, g/kg DM |
57.86b |
87.58a |
54.21b |
5.45 | <0.001 |
| Propionic/A, g/kg DM |
533.91b |
644.27a |
499.08b |
25.44 | 0.018 |
| Butyric/A, g/kg DM | 9.59 | 10.88 | 8.57 | 0.65 | 0.401 |
| Lactic/A, g/kg DM |
10.15b |
16.93a |
10.52b |
1.32 |
0.029 |
FJLB: fermented juice of epiphytic lactic acid bacteria, SEM: standard error mean
Different superscripts in the same row are significantly different at P < 0.05.
of FJLB could not decrease the pH value of silage to the acceptable range. It is because low addition rate of FJLB, 1% (v/w), was used in the process of silage making, which could not supply the require population of LAB to reduce silage pH even it has the highest LAB count among experimental silages.
The improvement of OM content with the addition of FJLB and molasses in this experiment is due to increase quality of fermentation (Harrison and Blauwiekel, 1994), thereby increasing population of LAB, improving quality of silage and avoiding the losses dry matter (McDonald et al., 1991), resulted higher OM content. Generally, the CP, NDF and ADF content were decreased with molasses addition, agreed with the report of Hill et al. (2001), and Huisden et al. (2009), they found that increase addition rates of molasses in silage making and long-term fermentation reduced the CP, NDF and ADF content compared with control. McDonald et al. (1991) explained that molasses is silage additive which contain readily fermentable carbohydrates, resulted to decrease ammonia-N by stimulating fermentation. Moreover, molasses is a stimulant of silage and caused to increase analysis in cell wall (Baytok et al., 2005), thereby decreasing fibre content of silage. Huisden et al. (2009) also stated that the NDF concentration decreased after a long fermentation, which may be due to the enzymatic or acid hydrolysis of the cell wall fraction.
The calculations of nutritional parameters such as TC, NFC, DMI, TDN and DMD related to NDF and ADF. The greater NFC, TDN, DDM and DMI in molasses silage are the result of the lower NDF and ADF content of that silage. These findings were consistent with the reports of (Bureenok et al., 2012); feed intake and digestibility of Napier grass were improved with the addition of molasses. Moreover, addition of molasses also improved the nutritional qualities of cassava leaves silage (Lukkananukool et al., 2018).
The acetic, propionic and lactic acid concentrations were enhanced with the addition of FJLB in silage making, however no changes on butyric acid concentrations were observed in this experiment. Moreover, among organic acid concentrations, the propionic acid is the highest and the lowest is butyric acid. Acetic acid concentration is also higher than lactic acid in this experiment. Regarding LAB count, the highest value was observed in FJLB addition silage. Shao et al. (2004) and Bureenok et al. (2005) reported that acetic acid is the primary fermentation acid in silages made from tropical grass. Kyawt et al. (2014) also stated that the higher percentage of acetic acid than lactic acid was observed in the cassava leaves silage. There are two evident for these results. Firstly, the population of lactic acid bacteria had changed from homolactic to heterolactic during the changed fermentation (Shockey et al., 1988). Secondly, when the fresh material had low level of WSC content or even no more available carbohydrate, LAB was able to utilize lactic acid and produce more acetic acid (Lindgren et al., 1990).
The NH3-N or VBN works as an important indicator of proteolytic activity during the fermentation process. The VBN content of control silage is higher than FJLB and molasses silage additives, indicating degradation of the protein fraction of silage materials. The reason for this finding is due to the different CP contents of experimental silages, the highest CP contents were observed in control and then followed by molasses and FJLB silage additives. Haaland et al. (1982) reported that the higher protein content of feed provided the higher NH3-N concentration as a result of the increasing proteolytic activity. Kyawt et al. (2014) also reported that NH3-N content in FJLB-treated silage was lower than that of control silage in ensiling of cassava leaves.
The VBN and V-score are the indicators for the determination of silage quality, which are negatively related. Thus, the good quality silage generally possessed the lower VBN and the higher V-score values. The over 80 points of V-score value indicated the good quality silage, whereas the range from 60 to 80 mean the normal silage, and below 60 points is for the low-quality silage (JGFFSA, 1994). The FJLB and molasses silage additives generally possessed lower VBN value and higher V-score compared with control, however their scores are lower than 60 points, indicating that they are low quality silages.
Conclusion
In general, the physical characteristics and nutritive values of molasses silage were higher than others, whereas the greater organic acid concentrations were observed in FJLB silage additives. The higher LAB count was found in FJLB and molasses silages. The low VBN and high V-score, indicators of good quality silages, were generally observed in molasses and FJLB silage additives rather than control. However, the V-scores of FJLB and molasses were lower than 60 points, specified low quality silage. Thus, this experiment indicated that FJLB can be alternatively used as additive instead of using molasses in sorghum silage; however more inclusion level of molasses and FJLB in ensiling of sorghum should be used to ensure good quality silage.
Acknowledgement
The authors would like to thanks Academic Melting Pot project from Faculty of Agricultural Technology, King Mongkut’s Institute of Technology Ladkrabang project for their funding assistance to conduct this experiment.
Conflict of interest
There is no conflict of interest.
AUTHOR’S CONTRIBUTION
AL, KS, AP, MA and YYK designed this experiment and, AL and YYK mainly carried out sample collection and silage making. AL, KS, AP and YYK analyzed the chemical compositions and silage quality. MA performed data analysis and interpretation. AL drafted the manuscript and KS, AP, MA and YYK completed the critical revision of the article. All authors read and approved the final version of manuscript.
References





