Advances in Animal and Veterinary Sciences
Research Article
Detection of Fungi Associated with Ocular Infections of Bovine
Ahmed A. Alkhalidi1, Osama F. Atshan1, Maisaa G. Taher2*, Zahid I. Mohammed1
1Department of Public Health, College of Veterinary Medicine, University of Diyala; 2Department of Pathology, College of Medicine, Diyala University, Iraq.
Abstract | In order to determine the fungi that association with ocular infections of bovine in Diyala/ Iraq. Eighty samples were collected from bovine which shows clinical signs of inflammation of the eye in the calves and cows are profuse Altadma, watery, serous and purulent thick secretions of the eye, swelling eyelids, adhesion eyelashes, keratitis with Keratohelcosis, congivetitis keratitis, emaciation and weakness. These samples were collected during four months, which began in January 2018 till April, from several areas of Baqubah city these samples were collected by sterile cotton swabs from the eyes and conjunctiva sac. The results showed that (45%) bovine samples were positive fungal isolation. The result showed higher percentage of fungal isolated in February(30%) followed by January(35%), March(25%) and April showed lower percentages of fungal isolates(10%).The current results explained that most of eye infections were unilateral (80%) while the bilateral were (20%). In conclusions, the fungus is one of the most important reasons that lead to eye infection and inflammation. The main fungal species that isolated were Aspergillus spp.(50%), followed by Mucorales also isolated (22.3%) then Candida spp. (16.6%), as well as the lower percentage of Alterneria spp. (11.1%).
Keywords | Fungal, Eye infection, Aspergillus, Candida, Alterneria.
Received | April 05, 2019; Accepted | May 03, 2019; Published | May 30, 2019
*Correspondence | Maisaa G Taher, Department of Pathology, College of Medicine, Diyala University, Iraq; Email: m_ghany2011@yahoo.com
Citation | Alkhalidi AA, Atshan OF, Taher MG, Mohammed ZI (2019). Detection of fungi associated with ocular infections of bovine. Adv. Anim. Vet. Sci. 7(7): 604-608.
DOI | http://dx.doi.org/10.17582/journal.aavs/2019/7.7.604.608
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Alkhalidi et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Fungi are widely prevalent in environment, which live outdoors in soil, water, air, sewage, grains, litter, vegetables, fruits and on trees as well as on many indoor surfaces and on animals skin. Some types of fungi can be harmful to animals health. (Pal, 2017).
The pathogenesis of the fungal eye disease is inextricably linked to the epidemiology. The pathogenesis of the fungal eye disease is inextricably linked to the epidemiology. It is worthwhile to state several proposed pathogenetic principles of fungal eye disease when discussing the epidemiology of fungal eye infections. The endophthalmitis attributed to infect sustained fungemia with even saprophytic fungi. (Klotz et al., 2000).
The eye diseases in large farm animals, especially ruminants including cattle, buffalo, sheep, goats, recorded a high rate in recent years as a result of lack of attention to these conditions and for the role played by the factories and its waste as a source of exciting and scarification of the eyes (Handool, 2013).
Eye diseases include conjunctivitis, keratitis, most frequently in horses (Ledbetter, 2017) and congestion cornea and abscesses under the conjunctiva, also inflammation of the eye Cause excitement and temporary blindness and permanent blindness and then affect on the length of grazing, which in turn affects on the growth and weight gain in developing animals and weight loss in adult animals and the occurrence of the loss after showing weakness and wasting (Ruehl et al., 1993; Handool, 2013).
Many factors, such as environment, age, geography, habitat and husbandry have been reported to influence the composition of conjunctival flora (Sgorbini et al., 2010; Johns et al., 2011).
In recent studies to isolate and identify conjunctival bacterial and fungal The most frequent fungal isolates were saprophytic fungi (Bourguet et al., 2019) in rabbits, (Khosravi et al., 2014) in horses, in addition of human (Shivaji et al., 2019; Ge et al., 2019). The aims of the present paper were to identify and quantify the fungi in clinically normal cattle and determine the percentage of fungal species associated with ocular infections of bovine.
MATERIALS AND METHODS
The Bovine Samples Collection
Eighty samples were collected from bovine which shows clinical signs of inflammation of the eye in the calves and cows are profuse Altadma and watery and serous and purulent thick secretions of the eye, swelling eyelids, adhesion eyelashes, keratitis with Keratohelcosis, congivetitis keratitis, emaciation, weakness. These samples were collected during four months, which began in January 2018 till April, from several areas of Baqubah city these samples were collected by sterile cotton swabs from the eyes and conjunctival sac.
All these samples were transmitted under aseptic conditions to the laboratory in the college of veterinary medicine / University of Diyala.
Preparation of Cultural and Biochemical Media:
(1) Sabouraud Dextrose agar (SDA): The medium was prepared according to the manufacturers’ directions (CM0139, Oxoid,
Basingstoke, Hampshire, England).
(2) Sabouraud Dextrose broth (SDB): The medium was prepared according to the manufacturers’ directions (CM0139, Oxoid,
Basingstoke, Hampshire, England).
Samples Culture
Each sample from bovine were cultured directly on three sabouraud dextrose agar media with chloramphenicol, incubated in the incubator at 28±2º C to assist growth of moulds and yeasts for (1-10) days with intermittent observation of the fungal growth, and when the growth appeared and complete the identification test was done according to Martin (1950).
Identification of Fungi
Mould identification
Macroscopic examination: The colonies morphology including shape, color, texture, consistency and reverse plate color and other apparent characteristics of the colonies were examined according to (Raber and Fennel, 1977).
Microscopic examination: One drop of lacto phenol cotton blue stain had been put on the slide and then mixed with a colony of mould then covered with a cover slip and examined under 40X lens to determine the shape of mycelium and shape of spores according to (Koneman et al., 1979, Pitt and Holking, 1997). Statistical analysis was applied by Nick TG (2007).
RESULTS AND DISCUSSION
Fungal Isolation and Identification
The results showed clear fungal colonies at 1-3 days post incubation on Sabouraud dextrose agar with chloramphenicol at 26-29ºC for yeast and moulds growth . The colonies were variable in their colours and shapes, microscopically, lactophenol cotton blue stain revealed mycelium, fruiting head, macro and microspores and chlamydospores, these results were agree with Samson and Van Reenen, (1988) who explained that the best temperature for yeast and moulds growth were 30ºC and 25ºC respectively.
We demonstrated that (45%) of bovine samples were positive fungal isolation and these isolates were variable according to months in which samples were collected, and the results showed high percentage of fungal isolated in February(35%) followed by January(30%), March(25%) and the lower percentage (10%) of fungal isolates at April (Figure 1).
The current results explained high percentage of fungal isolates in the cold months may be due to the crowding and multitude animals in the stockyards which lead to increase direct attachments and trauma with injury among them (Figure 1).
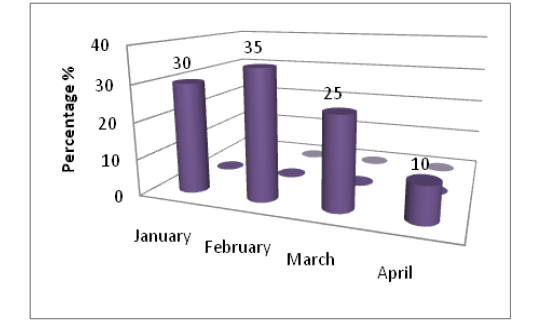
Figure 1: The percentage of positive samples with fungal during every month of study to total infected samples of eyes
The present study explained high percentage of fungi that isolated from eyes infection. Particularly if plant material such as a stick or a thorn caused the eye injury, some fungi in the environment associated with plant material there for Fungi can enter the eye and cause infection after an injury (Thomas et al., 2013). This evidence of eye infection attributed to locally infection more than systematically (Shamoon et al., 2006).
Positive cultures were obtained from 45% eyes for fungi. A total of 4 types of fungi were isolated. The current results explained that most of eye infections were unilateral (80%) while the bilateral were (20%), also the most frequent fungal isolates were saprophytic fungi (Figure 2).
The main clinical signs in affected animals that excessive lacrimation in eyes attacked by the different Pathological causes. Usually lacrimation starts as unilateral sign but later it becomes bilateral when both eyes are affected. most likely due to pasture grazing where these animals are exposed to long grasses, dusty environment which are predisposing factors for eyes infected (Swai et al., 2012). Eye diseases develops most frequently following the traumatic implantation of the infecting microorganism. cattle protruding eyes and lateral localization may be a risk factor that contributes to their predisposition to these infections (Sherman et al., 2017).
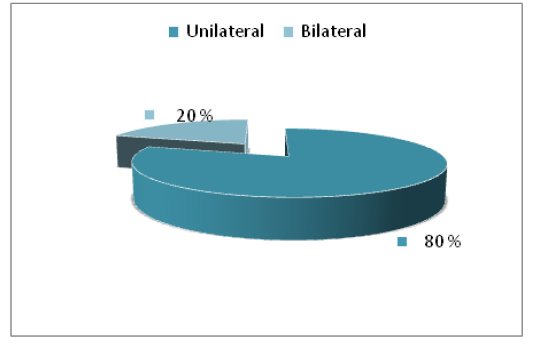
Figure 2: The percentage of infection site of eyes
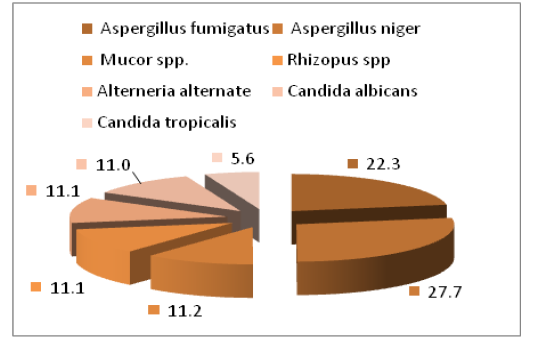
The main fungal species that isolated were Aspergillus spp (50%) which included Aspergillus niger (27.7%) and Aspergillus fumigatus (22.3%), then followed by Mucorales also isolated ( 22.3%) which include Mucor spp. (11.1%) and Rhizopus spp. (11.2%), Candida spp. (16.6%) especially C. albicans (11.0%) and C. tropicalis (5.6%), as well as the lower percentage (11.1%) of Alterneria alternate (Figure 3 and Table 1).
The fungal isolates were almost the same as with studies performed in other countries, although differences in species isolated could be related to geographic and climate difference.
The results of our study agreed with the (Samuelson et al., 2010) who isolated fungal species of Aspergillus and were similar to what has been isolated in this study. The present revealed that Aspergillus spp were constitute the 1st fungal isolates (50%), these finding was in similar with (Chihaya et al., 2001) who found that the Aspergillosis was found in (52.6%) calves, also with (Shamoon et al., 2006) who found that the Aspergillus niger was 62.5% of eyes infection in cattle and sheep.
Table 1: Percentage of fungal isolates from Ocular infections of Bovine
| Isolated fungal | Percentage |
| Aspergillus spp. | 50% |
| candida spp. | 16.6% |
| Mucorales spp. | 22.3% |
| Alternaria spp. | 11.1% |
| Total | 100% |
Mucorales also isolated in both species Mucor and Rhizopus may indicated that the mucormycosis form a third etiological agents of bovine fungal infections. The Mucormycosis is a disease animals caused by coenocytic fungi of the order Mucorales and present in animals feed (Abdual-shahid et al., 2013). Differences in fungal prevalence may be due to different methods of managing herds, though further studies are required to verify this hypothesis (Bonelli et al., 2014).
The current results explained that C.albicans constitute high percentage of fungal isolates, these observations may be indicated that C.albicans form important cause of bovine mycotic infection. Animal Candidiasis induce economic problems in dairy cattle via cause of abortion and mastitis, in addition, Candida albicans induce depression of immune response through its mannan cell wall which have ability to suppress cellular immunity as well as C.albicans avoid host defense as a result of antigenic variation on its cell surface (Ali and Khan, 2006). There for the animals may get infection systematically, fungal eye infections can happen after a fungal bloodstream infection such as candidemia spreads to the eye. these investigation are supported the result that mentioned by Astrid et al. (2011). As well as the lower percentage of, Alterneria alternate.
Conclusions
Fungal cause different pathological effects on eyes of bovine. This study proved that fungus is one of the most important reasons that lead to eye infection and inflammation. It was observed that several fungal genera were associated with the infected eyes of cattle.
Acknowledgement
The authors thankful all members of Department of public health college of veterinary medicine, University of Diyala for supporting with essential requirements for present research.
Conflict of Interest
Authors declare that they have no conflict of interest.
Authors Contribution
All authors contributed equally to the manuscript.
References