Advances in Animal and Veterinary Sciences
Research Article
Preliminary Study of Seroprevalence of Border Disease Virus (BDV) Among Sheep and Goats in Mosul City, Iraq
Hasan Sadam Dhahir, Al-Obaidi Qaes Talb*, Mohammed Hadeel Asim
University of Mosul, College of Veterinary Medicine, Department of Internal and Preventive Medicine, Mosul, Iraq.
Abstract | Border disease is one of the serious viral disease responsible for worldwide significant economic losses in sheep and goats. The objectives of the current study were to verify the seroprevalence of border disease virus (BDV) using indirect ELISA-kit, to investigate the persistent infection (PI) using the antigen ELISA (Ag-ELISA) kit for local breeds of sheep and goats in Mosul city. A total of 364 blood samples (264 sheep and 100 goats) of 20 local Awassi sheep and Shami goat herds with ≥1.5 years old age were collected from different regions of Mosul city. The sampling areas have no vaccination history against BDV, The seroprevalence of BDV was significantly higher in sheep 46.9% (124/264) than in goats 16% (16/100) (P<0.05). The prevalence of PI of border disease in sheep was 1.4% (2/140), but not detected in goats. This is a preliminary study of BDV in Mosul city, indicating that BDV is highly prevalent in sheep and goats of this region.
Keywords | Border disease, Seroprevalence, Persistent infection, ELISA.
Received | January 09, 2019; Accepted | April 23, 2019; Published | May 15, 2019
*Correspondence | Al-Obaidi Qaes Talb, University of Mosul, College of Veterinary Medicine, Department of Internal and Preventive Medicine, Mosul, Iraq; Email: qaes_talb@yahoo.com
Citation | Dahhir HS, Talb OQ, Asim MH (2019). Preliminary study of seroprevalence of border disease virus (bdv) among sheep and goats in mosul city, iraq. Adv. Anim. Vet. Sci. 7(7): 566-569.
DOI | http://dx.doi.org/10.17582/journal.aavs/2019/7.7.566.569
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Daher et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
The Border disease (BD) was first time reported by Hughes in sheep and goats in the border area between Wales and England in 1959. Thereafter the disease has been reported world-wide (Hughes et al., 1959). Border disease virus (BDV) belongs to the family Flaviviridae, genus Pestivirus (Fauquet et al., 2005). The virus infects a wide range of animals but mainly sheep and goats (OIE, 2017) and shares the genus with classical swine fever virus and bovine virus diarrhea viruses 1 and 2 (Oguzoglu et al., 2009). Numerous studies confirm that Pestiviruses are not highly host-specific. Accordingly, BDV infects sheep, swine, goats, cattle and also able to infect deer and giraffe (Paton, 1995, Oguzoglu et al., 2010). Depending on the presence or absence of the pathological changes produced by the virus during growth in cell culture, the virus is classified into two biotypes: non cytopathic and cytopathic (Tabash et al., 2009). Recent reports have recognized 1-7 genotypes depending on sequence alignment and phylogenetic analysis indicating the great multiplicity of various BDV genotypes (Giammarioli et al., 2011; Valdazo-Gonza et al., 2006). The transmission of BDV occurs either horizontally or vertically. The vertical transmission has a significant role in the epidemiology of the disease. The infection of fetuses in early pregnancy lead to the birth of persistently-infected lambs that turn out to be robust exporters of infection ensuring the transmission of the virus through secretion and excretion to susceptible animals (Monies et al., 2004; Fulton et al., 2005). Sheep infection with BDV is characterized by barren ewes, abortion, stillbirth and birth of small, weak lambs that exhibit tremor, abnormal and poor hair fleece quality referred to as “hairy shaker disease” or “fuzzy lamb syndrome” (Thabti et al., 2005; Oguzoglu et al., 2009). Affected lambs may have low birth weight, ataxia and occasionally enteric dysfunction and mucosal disease-like lesions have been also been recorded in sheep and lambs infected with BDV (Berriatua et al., 2006; Oğuzoğlu et al., 2008). In goats, abortion is the main clinical signs in pregnant animals and adult sheep or goats do not show clear signs of the infection (Thabti et al., 2005). Serological investigations have revealed that the prevalence of BDV varies from 5 to 50% according to the country or region investigated (Cabezon et al., 2010). Many researches have reported the prevalence of BDV in numerous countries including Turkey, Austria, Japan and Italy (Ataseven et al., 2006; Krametter-Froetscher et al., 2008; Giangaspero et al., 2011; Giammarioli et al., 2015). In Iraq, specific antibodies were detected against the causative agent of BDV in sheep in six governorates: Baghdad, Babel, Anbar, Salah Alden, Najaf, and Karbala (Al-Rubayie & Saleem, 2014).
In Mosul city, to date, there is no seroprevalence survey of BDV in sheep and goats. Therefore, this study aimed to ascertain the seroprevalence of BDV and to investigate the persistent infection (PI) in sheep and goats in Mosul city.
MATERIAL AND METHODS
Animals and Sampling
In the period between October 2018 and March 2019, the samples were obtained from different regions with different management systems including Gogjalee, Abo jarboaa, Al- Hamdanyah, Bartilla, and Bashiqa represented by 20 local Awassi sheep and Shami goat herds (≥1.5 year old) and no vaccination history against BDV in Mosul city, Iraq.
The required number of samples to study the status of the seroprevalence of BDV with absolute error 5%, confidence level 95% and expected prevalence of 30% based on earlier studies in different governates in Iraq (Al-Rubayie & Saleem, 2014), was at least 322 ewes and goats. The following relevant formula for random sampling was used:
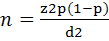
where n represents the number of sampled animals and Z is the value of the normal distribution for a 95% confidence level, P represents the expected prevalence, and d is the absolute error (Thrusfield, 2005).
A collection of 364 blood samples was obtained from apparently healthy animals comprising 264 local sheep and 100 local goat herds from private production farms (the herd sizes were ≥ 40 in sheep and ≥ 15 in goats). The blood samples were extracted from the jugular vein using 18G needle and kept in sterile vacutainer tubes without anti-coagulant for serum, which were separated by centrifuging at 2500 rpm for 15 min and stored at -20°C until laboratory analysis (Coles, 1986).
Laboratory Analysis
In this study, the commercial indirect ELISA-kit (SVANOVIR® BDV-Ab, SE-751 45 (Uppsala, Sweden), was applied to detect the antibodies against BDV in serum samples according to the manufacturer’s instructions and validated protocol. Persistently-infected animals were determined by using commercial test of IDEXX BVDV Ag/Serum Plus Test (IDEXX Laboratories, Inc. USA) complying with the manufacturer’s instructions. The plates of the test were fixed on ELISA plate reader and the optical density (O.D) was read at wavelength 450 nm by automatic plate reader (BioTek® Elx800, USA).
Statistical Analysis
Data analysis was performed using Chi-Square statistical method in IBM SPSS statistics 19 (SPSS Inc.).
RESULTS
From the total of 364 serum samples (264 ewe and 100 goats) tested for antibodies against BDV, 47% (124/264) and 16% (16/100) of the samples were positive for BDV in sheep and goats, respectively (Table 1).
Table 1: Seroprevalence of BDV in sheep and goats based on indirect ELISA test.
| Type of test | Type of animal | No. of samples tested | Negative samples (%) | Positive samples (%) |
| Indirect ELISA | Sheep | 264 | 140(53%) |
124 (46.9%)a |
| Goats | 100 | 84(84%) |
16(16%)b |
Significant differences (P < 0.05) between tests were labeled with different letters (a or b).
For the purpose of investigating the PI animals, the seronegative samples from sheep and goats in first test were retested against structural glycoprotein Erns antigen using monoclonal antibodies (MAbs) ELISA kit. The study showed that 2 from 140 (1.4%) were antigen positive for sheep while no antigen positive animals were detected in goats (0.0%) (Table 2).
Table 2: Prevalence of persistent infection in sheep and goats based on direct ELISA test.
| Type of test | Type of animal | No. of samples tested | Positive samples (%) |
|
Direct ELISA |
Sheep | 140 | 2 (1.4%) |
| Goats | 84 | 0(0%) |
DISCUSSION
This study was done to ascertain the seroprevalence of BDV in randomly-sampled sheep and goats in Mosul city in Iraq. The study revealed that the seroprevalence of BDV in sheep was 46.9% that indicated a high infection rate as there is no vaccination against BDV. This result was higher than a previous study done in other governates in Iraq that revealed 30.35% positive percentage, since there is no practice vaccination programs against BDV in Iraq, the differences in the prevalence between governate could be due to the regional, herd size and management systems differences (Al-Rubayie & Saleem, 2014). The prevalence of BDV in other neighboring countries was reported with higher or less seropositivity. It was 68.13% in Iran (Shohreh et al., 2014); 45.2% in Turkey (Mohammad Ameen et al., 2018), and 39.1% in Sudan (Ali et al, 2013). This study showed that the seroprevalence of BDV in goats was 16%, which was first time reported in Iraq. In Sudan it was 14.8% while in Turkey it was 63.6% (Ali et al, 2013; Mohammad Ameen et al., 2018). The reason for the differences in seroprevalence could be factors such as herd size, management of the farm, regional differences, sensitivity of the tests, uncontrolled animal movement, interspecies transmission and climate characteristics (Dubois et al., 2008; McFadden et al., 2012). The seroprevalence of BDV has been found to be considerably higher in sheep than in goats, it probably attribute to regional differences and/or larger sheep population in Mosul city. These findings are in agreement with Shohreh et al. (2014), Ataseven et al. (2006), Hasircioglu et al. (2009), and Ozan et al. (2012) who reported that the seroprevalence of Pestivirus between 18.94 and 90.27% in sheep, and between 5.7 and 63.6% in goats in different regions of Turkey. Our study confirmed the prevalence of persistently-infected animals was 1.4% in sheep, that may play an important role in the transmission of the virus and so high seropositivity rates among sheep, This finding also agree with (Shohreh et al. 2014). The percentage of persistently-infected sheep or viremic sheep ranges between 0.3% and 20% (Gür, 2009). In the current study no persistent infection was recorded in goats, probably due to the number of samples taken. This result agrees with the results of previous studies that revealed rare persistent infection in goats and abortion as major sign (OIE, 2017; Valdazo-Gonzalez et al., 2006).
In conclusion, the findings of this study indicate high prevalence of BDV among sheep and goats in Mosul city, Iraq. It is the first report of BDV in goats in Iraq. This study also shows the presence of persistently-infected sheep suggesting their role in maintaining and transmission of BDV. Our study recommends more comprehensive studies on Pestivirus infections in small and large ruminants in Mosul.
ACKNOWLEDGMENT
The authors are indebted to the college of veterinary medicine, Mosul University, Iraq for their financial support. The authors would also like to thank all animal owners for their invaluable cooperation in this study.
CONFLICT OF INTEREST
Authors declare no conflict of interests of the manuscript.
AUTHORS’ CONTRIBUTION
All authors contributed substantially to this study and are in full agreement with the content of the manuscript.
REFERENCES





