Advances in Animal and Veterinary Sciences
Research Article
Bacteriological and Histopathological Evaluation of Infectious Lymphadenitis Caused by Pseudomonas aeruginosa in Awasi Sheep
Wessam Monther Mohammed Saleh1*, Mohanad H. Lafta1, Ashraff Waleed Abdulrazaq2, Hassan Nima Habib3, Luay A. Naeem2
1Department of Internal and Preventive Medicine, College of Veterinary Medicine, University of Basra, Iraq; 2Department of Surgery and Obstetrics, College of Veterinary Medicine, University of Basra, Iraq; 3Department of Animal Production, College of Agriculture, University of Basra, Iraq.
Abstract | Infectious lymphadenitis in small ruminants which caused by broad ranges of microorganismsis worldwide distributed disease, that subsequently causing significant economic losses to animal production industry. Clinical cases of ovine lymphadenopathy in Awasi sheep are described in this research. These cases were recorded separately in the animal’s farm of College of Agriculture, University of Basra / Iraq during November 2017. A 2-4year-old Awasi sheep weighing 30-40 kg, presented to the Large Animal Clinic, College of Veterinary Medicine, University of Basra with an enlargement of superficial lymph nodes. Surgical removals of the affected lymph nodes were done and samples were processed to histopathological and bacteriological examinations. The sheep were tentatively diagnosed with Infectious lymphadenitis. The present study revealed that the infectious lymphadenitis in Awasi sheep were caused by Pseudomonas aeruginosa and the infected sheep might provide evidence for disease transmission and source of infection in the farm as well as human.
Keywords | Awasi sheep, Histopathology, Infectious Lymphadenitis, Microbiology, Pseudomonas aeruginosa.
Received | December 16, 2018; Accepted | January 16, 2019; Published | March 02, 2019
*Correspondence | Wessam Monther Mohammed Saleh, Department of Internal and Preventive Medicine, College of Veterinary Medicine, University of Basra, Iraq; Email: wessamgm@gmail.com
Citation | Saleh WMM, Lafta MH, Abdulrazaq AW, Habib HN, Naeem LA (2019). Bacteriological and histopathological evaluation of infectious lymphadenitis caused by pseudomonas aeruginosa in awasi sheep. Adv. Anim. Vet. Sci. 7(5): 378-382.
DOI | http://dx.doi.org/10.17582/journal.aavs/2019/7.5.378.382
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Saleh et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Lymphadenopathy is currently still a major medical issue for a large number of patients, the causes of this condition are still undiagnosed (Safont et al., 2014). In literature, chronic suppurative lymphadenitis of visceral and superficial lymph nodes in small ruminants have been reported as an “abscesses disease” and “caseous lymphadenitis”(Al-Harbi, 2011; de la Fuente et al., 2017; Gururaj et al., 2018). Caseous lymphadenitis is a chronic bacterial disease which caused mainly by Corynebacterium pseudotuberculosis, it is responsible for a worldwide significant economic losses in sheep and goat breeding (Umer et al., 2017). As a broad range of the causative agents of infectious (caseous) lymphadenitis in sheep and goats, the differential diagnosis should not include only the main classical causes such as Corynebacterium pseudotuberculosis and Staphylococcus aureus subspecies anaerobius. Moreover, other microorganisms such as Pseudomonas aeruginosa, Pseudomonas hydrophila, Treuperella pyogenes, Staphylococcus lentus, Actinomyces hyovaginalis, Streptococcus ovis, Staphylococcus simulans, Staphylococcus equorum, Staphylococcus capraeh, Staphylococcus warneri, Corynebacterium camporealensis, Vagococcuslutrae Actinobacillus pleuropneumoniae, and Acinetobacter spp should also be included (de la Fuente et al., 2017). Nevertheless, because of the way that more than one of these organisms are as often as possible found in a same flock, it is important to analyze specimens from several animals to achieve an exact conclusion in flocks influenced by lymphadenitis (Al-Harbi, 2011; de la Fuente et al., 2017). Chronic suppurative mandibular and maxillary osteomyelitis are also another complications of Pseudomonas aerurginosa infection in sheep (Rasooli et al., 2018). The treatment for this illness is not powerful, and an exceptional vaccination timetable would be the best control system (de Oliveira Silva et al., 2018). Thus, accurate diagnosis of caseous lymphadenitis is what preoccupied researchers over the last decades. There is no unique diagnostic test that could recognize all cases or even different phases of caseous lymphadenitis (Oreiby, 2015). Little is known on the etiological diversity and thepathogenicity of infectious lymphadenitis in Iraq.Therefore in this report, we described the cases of infectious lymphadenitis in Awasi sheep bacteriologically and histopathologically, to amplifyits impact as a potential source of infection transmission of the disease to other susceptible animals as well humans.
Material and Methods
Animals and Sampling Protocol
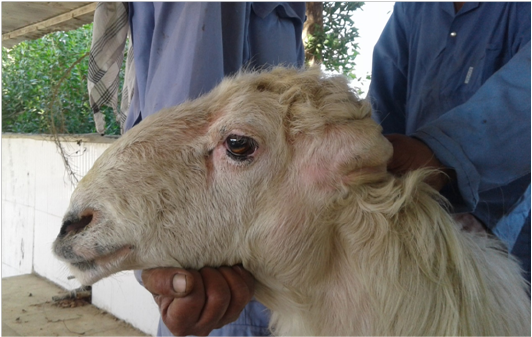
Cases of ovine lymphadenopathy in Awasi sheep were reported separately in the animal’s farm of College of Agriculture, University of Basra, Basra City, Iraq during November 2017. A 2-4 year-old Awasi sheep (n = 7), 3 males and 4 females weighing between 30-40 kg, presented to the Large Animal Clinic, College of Veterinary Medicine, University of Basra with an enlargement (hard mass) of one or moresuperficial lymph nodes including: retropharyngeal, mandibular and prescapular lymphnodes. Appetite and all the vital parameters were normal in all sheep.The farm’s management and also the clinicians had suspected this case as a caseous lymphadenitis from their experience. Surgical removals of the affected lymph nodes were done using local anesthesia and the samples were processed separately to histopathological and bacteriological examination. Our study was approved according to the guidelines and the roles of the Animal Researches Committee of College of Veterinary Medicine, University of Basra, Basra (Iraq).
Bacterial Isolation and Identification
Swabs that have taken from pus content of the affected lymph nodes were cultured separately on Nutrient and Blood agar cultures (Difco, USA) aerobically at 37°C for 48 hour in Central Research Unite, College of Veterinary Medicine / University of Basra. Bacterial colonies were recognized according to its morphological characteristics as well as biochemical activities including Diffusible Pigment Production, Catalase, Oxidase, Lipase, Nitrate reduction, Methyl red, Urea hydrolysis, Citrate, Indole, Gelatin, Glucose, Mannitol, Sucrose, Maltose, Lactose and Hemolysis in blood agar (Quinn et al., 2011). Smears were set up from the suspected colonies and stained with Gram’s for microscopic examination. However, identification of Pseudomonas aeruginosa isolates was confirmed on its production of greenish exopigments and oxidase compound distinguished by freshly prepared“1% α-naphthol” in 95% ethanol and a “1% aqueous solution of P-Aminodi methylaniline oxalate”.
Histopathology
Histopathological procedures were done to the lymph nodes samples according to procedure of Luna, 1996 (Luna, 1968). Parts of the affected lymph nodes were placed for at least 24 hours in 10% Formalin buffer for fixation. Then all samples were processed using an automatic tissue processor, embedded in paraffin wax and cut at 4 µm. The tissue sections were placed on glass slides, stained with Hematoxylin and Eosin and covered with a drop of DPX and cover slip. Score of lesions were done to assess the severity of the histopathological changes. 20 slides of every lymph node were chosen and six microscopic fields were analyzed under a light magnifying lens utilizing 200X amplification. The scoring framework comprised of four scores where: 0= normally (no obvious lesion), 1= mild (less of 33% of the field included), 2= moderate (up to 66% of the field was included) and 3= severe (if multiple thirds of the field was included).
Statistical Analysis
In the current study, “Microsoft EXCEL” software was used for evaluating the average of the histopathological changes scores while “Chi Square Test” was used to analysis the results of the biochemical tests.
Table 1: Results of the biochemical characterization of P. aeruginosa
| Gram Stain | Pigment | Catalase | Oxidase | Lipase | Nitrate reduction | Methyl red | Urea hydrolysis | Citrate |
| - | Bluish green | + | + | + | + | - | - | + |
| Indole | Gelatin | Motility | Glucose | Mannitol | Sucrose | Maltose | Lactose | Hemolysis |
| - | + | + | + | + | - | - | - | Beta |
Table 2: Histopathological score of lymph nodes
| Animal | Lesion scoring of lymph nodes Mean ± S.E. | |||
| Inflammatory cell Infiltration | Congestion/ Hemorrhage | Necrosis/Degeneration | Edema | |
| 1 | 2.12±0.32 | 2.13±0.28 | 1.77±0.42 | 1.21±0.12 |
| 2 | 1.74±0.29 | 1.01±0.45 | 1.07±0.31 |
0.53±0.17 |
| 3 | 2.11±0.25 | 2.06±0.26 | 1.36±0.22 | 1.10±0.20 |
| 4 | 1.43±0.31 | 1.11±0.34 | 0.81±0.21 | 0.40±0.08 |
| 5 | 2.76±0.18 | 2.27±0.26 | 2.44±0.23 | 1.00±0.00 |
| 6 | 1.73±0.28 | 1.94±0.19 | 1.75±0.26 | 1.00±0.00 |
| 7 | 1.16±0.14 | 1.22±0.32 | 0.73±0.21 | 1.03±0.18 |
| Average | 1.86±0.20 |
1.68±0.20a |
1.42±0.23 |
0.90±0.12 |
Results
Pseudomonas aerugenosa infection led to induce pathological changes in the superficial lymph nodes of Awasi sheep; one or more lymph nodes were infected in each animal. However, all the seven tested cases had identified as infectious lymphadenitis due to P. aeruginosa infection based on the clinical manifestation, bacteriological and histopathological examinations.
Bacteriologically, pale flat serrated edge with grape-like odor colonies were identified in all swaps which cultured on Nutrient and Blood agar media. The bacteria were growth and survive when it were further incubated at 42⁰C. These findings were supported by the biochemical characteristic of P. aeruginosa (Table 1).
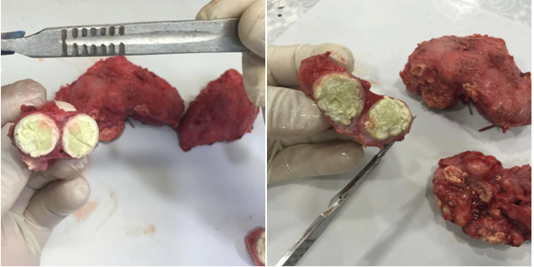
Macroscopically, marked enlargements (due to abscesses formations) of different size were observed in the superficial lymph nodes of the affected Awasi sheep. The common infected lymph nodes were the: retropharyngeal, mandibular and prescapular lymph nodes. Moreover, mild to moderate cellular changes have seen in the histological sections of the affected lymph nodes such as: edema, suppuration of lymphoid tissue, granulomatous inflammatory reaction, hemorrhage, congestion with neutrophils and macrophages infiltration (Figure 3). The mean of the cellular changes score of the affected lymph nodes is presented in Table (2).
Discussion
In the current study, gross and histopathological responses following natural P. aerugenosa infection were detected in

Figure 3: Photomicrograph of the affected lymph nodes of Awasi sheep infected with Pseudomonas aeruginosa showing: area of suppuration of lymphoid tissue (A), medullary area in which the sinusoids were rich in neutrophils and macrophages and severe congestion and edema (B), suppuration of lymphoid tissue, granulomatous inflammatory reaction and congestion (C), anddilated sinusoid (due to edema) in medulla with some macrophages and neutrophils (D) (H&E stain 200X).
the superficial lymph nodes of Awasi sheep. These irreversible changes could impair the functional effect of the lymphatic system as it is known to be responsible for the protection of the host against hostile external environment including of organisms and other foreign substances (Willard-Mack, 2006). Due to the opportunistic nature of P. aeruginosa and the worldwide spreading of the organism in water, soil, and on plants, skin, mucous membrane and in feces (Quinn et al., 2011), the organism was consequently gets entry to the lymphatic system and proliferate in the affected lymph nodeproducing a caseous lymphadenitis in Awasi sheep. In the present study, the histopathological feature of P. aeruginosa infection revealed different degrees of cellular changes such as: edema, suppuration of lymphoid tissue, granulomatous inflammatory reaction with inflammatory cells infiltration. Thus, the cellular changes which were observed in the infected lymph nodes could be as a result of the action of the “Phospholipase C” which is determined as the virulence factor of P aeruginosa (Safont et al., 2014). Furthermore, those infected lymph nodes have a suspicious role of further spreading of the organism to the herd and also to the persons who are in contact with the infected animal especially during shearing, vaccinating and breeding. Pseudomonas aeruginosa is responsible of several conditions such as; hepatic abscesses (Santa Rosa et al., 1989), abscesses disease, caseous lymphadenitis (Al-Harbi, 2011), fleece rot and dermatitis in sheep and goats (Constable et al., 2016). Human lymphadenopathy could be attributed by Pseudomonas aeruginosa (Safont et al., 2014). Therefore, early identification of this species is essential for epidemiological and control strategies whereas the treatment for the complications of P. aeruginosa infections is not effective due to widespread resistance to many first line antibiotics. To date, control of infectious lymphadenitis is generally unsuccessful, because of its tendency that leads to relapse the problem even whether a single case escapes determination (Oreiby, 2015). Therefore, an appropriate investigation of the etiological diversity of caseous lymphadenitis is of value for controlling measurements which subsequently leading to increase the efficacy of the vaccination and the eradication programs. However, the prevalence of infectious lymphadenitis of small ruminants in Iraq is not frequently known, and the economic with the zoonotic impact of the disease in this country are also undetermined.
In conclusion, our study illustrated the possibility of natural occurrence of caseous lymphadenitis in sheep due to infection with P. aeruginosa. It is worthy of noting that the histopathological changes due to P. aeruginosa infection are more common in lymph nodes which probably due to the liquefactive effects of Phospholipase C, a virulence factor of P. aeruginosa. Finally, future molecular studies should focus on phenotypic variability of this organism regarding to other closely related species. Further experimental studies should for more understanding the pathogenicity and the disease progression of this organism in small ruminants should also be needed.
Acknowledgements
I would like to recognize Prof Dr. Saleh K. Majeed for his aiding and direction in histopathological investigation, likewise an abundance of thanks send to all the staff of the Large Animal Clinic, College of Veterinary Medicine, University of Basra and to the staff of Animal Farm, College of Agriculture, University of Basra for their helping, direction and supporting enabling us to finish this work.
Conflict of Interest
No conflict of interests is declared by authors for the contents in this manuscript.
Authors Contribution
Wessam Monther Mohammed Saleh, Mohanad H. Lafta, Ashraff Waleed Abdulrazaq, Hassan Nima Habib and Luay A. Naeem have designed and carried out the experiment and prepared the draft manuscript.
References