Advances in Animal and Veterinary Sciences
Research Article
Effect of adding white tea powder (Camellia sinensis) to the Japanese quail (Coturnix coturnix japonica) birds rations in some traits of blood biochemical and liver enzymes
Fadhil Rasul Abbas1, Nihad Abdul-Lateef Ali1*, Imad Abdul-Jabar1, Ammar Salahaldeen AL- Nassry2
1College of Agriculture, Al-Qasim green University. Iraq; 2College of Agriculture, University of Tikrit. Iraq.
Abstract | The experiment was conducted in the animal production field that is following to College of Agriculture, University of Tikrit, and for 8 weeks to study the effect of adding white tea powder (Camellia sinensis) to the Japanese quail (Coturnix coturnix japonica) birds rations in some traits of blood biochemical and liver enzymes, the 45 females were used with age of 24 weeks. Birds were randomly distributed to three treatments, each treatment consists of five cages, where in each cage was placed 3 female quail and treatments were as follows: The first treatment: (T1) standard ration without addition, the second treatment: (T2) standard ration added 1 g white tea powder / kg feed, and the third treatment: (T3) standard ration added 1.5 g white tea powder / kg feed. The blood samples were collected after slaughtering the birds in tubes that did not contain the anticoagulant. The blood plasma was separated by a centrifuge at 3000 cycles for 15 minutes. The serums was kept in clean tubes at -20 °C. The experiment included the study of the following traits: Uric Acid, Glucose, Total Protein, Albumin, Globulin, Cholesterol, Triglycerides, High-Density Lipoprotein(HDL), Low Density Lipoproteins(LDL), Glutamic-Oxaloacetic Transminase Enzyme (GOT), Transminase Glutamic-Pyruvic Enzyme (GPT) and Alkalin-Phosphatase Enzyme (ALP). The results showed significant improvement (p <0.05) in white tea treatments in total protein concentration, albumin, globulin, high density lipoproteins and (ALP) compared to control treatment and significant decrease (p <0.05) in uric acid concentration, glucose, cholesterol, triglycerides, Low Density Lipoproteins, GOT and GPT. The current experiment suggests that the addition of white tea in the rations can lead to improvement of some parameters of biochemical blood and ALP enzyme.
Keywords | White tea, Biochemical blood traits, Liver enzymes, Quail.
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | September 01, 2018; Accepted | December 02, 2018; Published | December 29, 2018
*Correspondence | Nihad Abdul-Lateef Ali, College of Agriculture, Al-Qasim green University. Iraq; Email: agricnano.egypt.2017@gmail.com.
Citation | Abbas FR, Ali NAL. Abdul-Jabar I, Al-Nassry AS (2019). Effect of adding white tea powder (camellia sinensis) to the japanese quail (coturnix coturnix japonica) birds rations in some traits of blood biochemical and liver enzymes. Adv. Anim. Vet. Sci. 7(3): 205-209.
DOI | http://dx.doi.org/10.17582/journal.aavs/2019/7.3.205.209
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Abbas et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
It was expected that after the spread of manufactured chemical drugs and their variety that the diseases will decline and become more controlled, but what happened was the opposite has known modern human diseases were not known before and began chronic diseases to appear and spread, This may be due to the fact that many industrial drugs act to inhibit immunity as well as its long-term side effects that are finally emerging, while there are many plants are very effective in therapeutic uses, because each plant or herb is made up of a variety of effective substances placed by God Almighty in proportions so that they do not have serious negative side effects on human health (Tipu et al., 2006). Therefore, medicinal plants have taken a prominent place in global agricultural production because they contain natural chemicals of great interest and importance in their physiological influence and therapeutic activity of humans and animals. Scientific studies have shown that the products derived from these plants have the ability to heal from many diseases and remove their symptoms,as well as, stimulating it to digestive functions by increasing the production of digestive enzymes, enhancing the effectiveness of liver, pancreas and small intestine, forming the bile and activating its secretion. It also helps reduce fatty level in serum and improve immune state (Rahman and Lowe, 2006). White tea is one of the most common teas in developed countries; White tea is now occurring the first rank in terms of interest. Japan and China are the most prolific producing countries for white tea. It is one of the rarest tea varieties and is produced by picking small buds and small leaves for tea before turned into a green color. It is carefully cared for after the cut, drying it very carefully, This is what makes it the rarest of tea types and has the ability to revitalize the body and alert the mind and the heat-softening and resistant to thirst and is used in the treatment because it contains many chemical compounds effective such as compounds flavonoids, catechin form a higher proportion of them and it is also an effective antioxidant (Antan and Shella, 2003, Saffari and Sadrzadeh, 2004). White tea contains fluoride, and many antioxidants such as Polyphenole, methyl Xanthine, Theobromine, Caffeine, tannic acid, and many important compounds (Gargi Saha et al., 2017). Manganese is one of the most abundant minerals in white tea, which plays an important role as an adjuvant in the digestion of proteins and is very necessary for bone tissues and connective tissues (Costa et al., 2002; Wang and Ho, 2009). As well as it contains a good level of vitamins, especially vitamin E and C (Unachukwu et al., 2010). Since there is no research on the effect of white tea on poultry, the aim of this study was to find out the effect of adding white tea powder (Camellia sinensis) to the Japanese quail birds rations (Coturnix coturnix japonica) in some traits of biochemical blood and liver enzymes. This study is the first of its kind in Iraq on this type of plant medical.
MATERIALS AND METHODS
The experiment was conducted in the quail field that is belonging to the animal production department, College of Agriculture, University of Tikrit for 12/3/2018 until 6/5/2018 to study the effect of adding white tea powder to the Japanese quail birds rations in some traits of biochemical blood and liver enzymes, 45 females with 24 weeks age were used. Birds were randomly distributed to three treatments, each treatment consists of five cages made of iron clasp with three floors (measuring the cage 40 × 40 × 40 cm, where in each cage was placed 3 female quail and treatments were as follows: The first treatment: (T1) standard ration without addition, the second treatment: (T2) standard ration added 1 g white tea powder / kg feed, and the third treatment: (T3) standard ration added 1.5 g white tea powder / kg feed.The white tea was added to the feed manually. Table (1) shows active compounds in white tea. Water and feed were freely provided throughout the study period. At the end of the experiment, which lasted eight weeks, the blood samples were collected after slaughtering the birds in tubes that did not contain the anticoagulant. The blood plasma was separated by a centrifuge at 3000 cycles for 15 minutes. The serums was kept in clean tubes at -20 °C. Diagnostic kits of Jordanian origin for the measurement of cholesterol (mg / 100 ml) were used according to (Franey and Elias, 1968). The concentration of glucose was measured by using Kits from French origin according to the following equation:
Glucose concentration (mg/ml) = 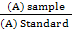 × standard concentration (100 mg/ml)
× standard concentration (100 mg/ml)
The triglycerides, low density lipoproteins and high density lipoproteins were estimated according to (AOAC, 1980), total protein (gm/100 ml) and uric acid (mg/ml) based on the method mentioned by (Henry et al., 1982); the concentration of Globulin was calculated according to the following equation:
Globulin concentration = total protein concentration - albumin concentration
Liver enzymes were estimated according to the method described in (Reitman and Frankle, 1957). Table (2) shows the feed material used and the calculated chemical composition during the experiment period.
Table 1: Assessment of Biochemical components of White tea (Gargi Saha et al., 2017).
| White tea (Aqueous) | White tea (Methanolic) | Biochemical Components |
| 29±.01 | 35±.01 | Total Polyphenols (%) |
| 13±.01 | 19±.01 | Catechins (%) |
| 1±.001 | 5±.001 | Total Flavonoids (gm/100gm) |
| 3.9±.01 | 4.8±.01 | Caffeine (gm/100gm) |
| 9±.001 | 11±.001 |
Tannins (%w/w) |
Completely Randomized Design was used to study the effect of different treatments in the studied traits, the differences between the averages were compared using the Duncan multidimensional Test (Duncan, 1955), and the statistical program SPSS (2008) was used for data analysis.
RESULTS AND DISCUSSION
Table (3) indicates the effect of the addition of white tea to the ration in some of the biochemical blood traits for the Japanese quail bird, breeding for 26 to 34 weeks. There were significant differences (P <0.05) between the treatments in concentration of glucose and uric acid (mg / 100 ml), The first treatment (control) gave the highest level by recording
Table 2: Primary feed materials used in the ration of Japanese quail bird during the experiment period with calculated chemical composition.
| Primary feed materials1 | Percentage (%) |
| Yellow corn grinded | 53.10 |
| Soybeans (48.5% protein) | 33.10 |
| Plant oil (sunflower) | 4.00 |
|
Premix1 |
2.50 |
| Limestone | 7.00 |
| Salt | 0.30 |
| Total | 100 |
|
The calculated chemical analysis2 |
|
| Representative energy (kCal / kg) | 2832.73 |
| Raw protein (%) | 20.50 |
| Raw fiber (%) | 3.62 |
| Calcium (%) | 2.89 |
| Phosphorus availability (%) | 0.40 |
| Lysine (%) | 1.12 |
| Methionine (%) | 0.47 |
| Methionine + Cicin (%) | 0.80 |
(BROMIX-2.5W) from the Dutch company WAFI contain of 1.6% Lysine, 6% methionine, 6% methionine + cystine, 23.2% calcium, 9.3% phosphorus, 4.9% sodium, 440000 IU / kg vitamin A, 120000 IU / kg vitamin D3, 1200 mg / kg vitamin E, 100 mg / kg vitamin K3, 120 mg / kg vitamin B1, 280, mg / kg vitamin B2, 160 mg vitamin B6, 1400 mg / kg vitamin B12, 600 mg / kg, 40 mg / kg Folic acid , 4 μg / kg bayutin, 2000 μg / kg iron, 400 microgram / kg copper, 3200 μg / kg magnesium, 2400 μg / kg zinc, 10 μg / kg selenium, 1200 mg / kg chlorine chloride.
According to the chemical composition according to the analysis of feed materials in NRC (1994).
it (169.476, 3.12 mg / 100 ml), respectively, while the third treatment (1.5 g / kg feed) showed the lowest value of glucose and uric acid concentration in blood plasma of (134.397, 3.02 mg / 100 ml), respectively, followed by the second treatment (1 g / kg feed) which recorded the concentration of glucose and uric acid was (156.319, 3.04 mg / 100 ml), with significantly different from the first treatment. As for the concentration of total protein (100 mg / ml), we note the second and third treatments was significantly excelled than the control treatment (the first) by giving them the highest concentration of (6.634, 7.047 g / 100 ml), respectively, while the first treatment recorded (5.857 g / 100 ml).The excelling of white tea treatments continued in the biochemical blood traits. The third treatment was significantly excelled in albumin and globulin (g/ 100 ml) concentration by giving it a value of (2,874, 4.158 g / 100 ml) respectively, compared to the first treatment, which recorded (2.434, 3.422 g / 100 ml), respectively, the second treatment gave the following values (2.614, 3.877 g / 100 ml), respectively, without significant differences from the first and third treatments.
The reason for the positive results obtained is that white tea may contain compounds similar to insulin or Insulin secretion activator. Bursill et al. (2001) and Kuhn et al. (2004) reported that green tea contains polysaccharides is substance that plays an important role in reducing the level of glucose in the serum.The results also showed that the addition of white tea to feed reduces the exposure of birds to any type of stress by increasing the secretion of thyroxine hormone, thus increasing the rate of speed metabolism and increase the body’s vital reactions and building muscle tissue in the body, which results in maintaining a high rate Of the total protein in birds blood of study parameters compared to control treatment. Table (4) indicates the effect of adding white tea to the ration in the concentration of cholesterol and fatty level in serum for the Japanese quail bird, breeding for 26 to 34 weeks, it is noted that the white tea treatments (second and third) have recorded A significant decrease in the concentration of cholesterol and triglycerides as well as low-density lipoprotein (mg / 100 ml) which gave the following concentrations of (175.67, 178.67 mg / 100 ml) cholesterol, (159.67, 162.00 mg / 100 ml) for triglycerides and (111.6, 123.3 mg / 100 ml) for low density lipoproteins, respectively, compared to the control treatment (first), which recorded the following concentrations (187.00, 178.33, 138.00 mg / 100 ml), respectively.As for the concentration of high-density lipoproteins, the second and third white tea treatments recorded the highest concentration (44.67, 40.67 mg / 100 ml) respectively, with a significant difference (P <0.05) from the control treatment (the first), which recorded the lowest concentration (36.00 mg / 100 ml).
The reason for the white tea’s ability to reduce the level of cholesterol is due to the presence of active substances in the extract such as Catechin and Epigallocatechin, which are powerful antioxidants, reducing the absorption of cholesterol and increasing the secretion of Bile salts where there is a virtual effect in inhibiting liver cholesterol industry (Friedman, 2007). This may be due to the ability of active substances in white tea to inhibit the β-hydroxy-β-methyl glutary-CoA reductase enzyme responsible for the construction of cholesterol (Bishop, 1999). Moreover, the presence of flavonoids in the white tea extracts plays an important role in lowering the level of cholesterol within the intestines by increasing its secretion in the Defecation and in turn reduces the level of cholesterol in the liver (Ekayanti et al., 2017). The decrease in the concentration of triglycerides in the addition of white tea may be due to the role of polysaccharides in white tea, which may have played a role in reducing the level of glucose in the serum and thus reduce the level of triglycerides by reducing the storage of Triglyceride In order to meet the birds needs of the energy (Bishop, 1999). This is due to the role of active
Table 3: The effect of the addition of white tea to the ration in some of the biochemical blood traits for the Japanese quail bird, breeding for 26 to 34 weeks (average ± standard error)
| Treatments/ Traits | The first treatment (control) | The second treatment (1 g white tea) | The third treatment (1.5 g white tea) |
| Glucose concentration (mg / 100 ml) |
169.476 ± 3.490 a |
156.319 ± 2.46 b |
134.397 ± 1.939 c |
| Uric Acid concentration (mg / 100 ml) |
3.12 ± 0.010 a |
3.04 ± 0.005 b |
3.02 ± 0.010 c |
| Total Protein concentration (g / 100 ml) |
5.857 ± 0.150 b |
6.634 ± 0.160 a |
7.047 ± 0.146 a |
| Albumin concentration (g / 100 ml) |
2.434 ± 0.135 b |
2.614 ±0.091 ab |
2.874 ± 0.138 a |
| Globulin concentration (g / 100 ml) |
3.422 ± 0.210 b |
3.877 ± 0.246 ab |
4.158 ± 0.102 a |
* The different characters in each column indicate significant differences between the average of the treatments at (p <0.05)
Table 4: The effect of the addition of white tea to the ration in the concentration of cholesterol and fatty level in serum for the Japanese quail bird, breeding for 26 to 34 weeks (average ± standard error)
| Treatments/Traits | The first treatment (control) | The second treatment (1 g white tea) | The third treatment (1.5 g white tea) |
| Cholesterol concentration (mg / 100 ml) |
187.00 ± 0.557 A |
175.67 ± 0.333 c |
178.67 ± 0.333 b |
| Triglycerides concentration (mg / 100 ml) |
178.33 ±0.333 A |
159.67 ± 0.333 c |
162.00 ± 0.577 b |
| High-Density Lipoprotein concentration (mg / 100 ml) |
36.00 ± 0.577 C |
44.67 ± 0.333 a |
40.67 ± 0.333 b |
| Low Density Lipoproteins concentration (mg / 100 ml) |
138.00 ± 0.577 A |
111.6 ± 0.333 c |
123.3 ± 0.006 b |
* The different characters in each column indicate significant differences between the average of the treatments at (p <0.05)
Table 5: The effect of the addition of white tea to the in the liver enzymes for the Japanese quail bird, breeding for 26 to 34 weeks (average ± standered error)
| Treatments/Traits | The first treatment (control) | The second treatment (1 g white tea) | The third treatment (1.5 g white tea) |
| Concentration of GOT enzyme (IU / L) |
99.03 ± 0.057 A |
97.63 ± 0.057 b |
91.23 ± 0.115 c |
| Concentration of GPT enzyme (IU / L) |
10.3 ± 0.152 A |
9.73 ± 0.057 b |
9.20 ± 0.100 c |
| Concentration of ALP enzyme (IU / L) |
186.3± 0.577 B |
187.67 ± 0.577 ab |
188.3 ± 1.155a |
* The different characters in each column indicate significant differences between the average of the treatments at (p <0.05)
compounds in white tea, especially flavonoids, which play an important role in reducing the level of cholesterol and fat within the intestines and increase their secretion in Defecation, also reduces the level of cholesterol in the liver and also reduce the levels of cholesterol (LDL- Cholesterol) (Ekayanti et al., 2017).The role of white tea in influencing the representation of fatty cholesterol groups may be due to flavonoids that play a protective role for the liver by stimulating the effectiveness of the Super Oxide Dismutase enzyme (SOD). It also activates the enzymes of the drug representation system, which is mainly in hepatic tissues and contributes to the protection system of cells and tissues from the crash due to the effect of free radicals with protection system existing within the body represented by the enzymes and antioxidant vitamins as an E and C vitamin (Huang et al., 1992). The role of white tea in the reduction of low-density lipoproteins and the increase in high-density lipoproteins is due to its high content of flavonoids (Adnan et al., 2013), especially Catechins, which are strong antioxidants. One study estimated that the strength of antioxidant in white tea about 100 times more than in vitamin E, and these antagonists may inhibit lipid peroxidation chain reactions (Hollman et al., 1999), Which scavenge the varieties of oxygen radicals and nitric.Table (5) shows the effect of adding white tea to the ration in the liver enzymes of the Japanese quail birds, breeding for 26 to 34 weeks. The first treatment (control) recorded the highest concentration of GOT and GPT, with significant (p <0.05) differences from the second and third treatments which recorded the following values (99.03, 10.3 IU / L), respectively. While the second and third treatments recorded the following values (97.63, 91.23 IU / L) respectively for the GOT and (9.73, 9.20 IU / L), respectively for GPT.As for the ALP enzyme, the third treatment was significantly excelled (p <0.05) on the first treatment (control) by giving it the highest concentration of ALP was (188.3 IU / L) followed by the second treatment, which recorded (187.67 IU / L), without significant differences, while the first treatment recorded the lowest concentration of ALP (186.3 IU / L). Almajano et al. (2008) showed that the reason for increased serum ALP may be due to active compounds such as flavonoids in white tea, which act to move metals from bone and increase the level of calcium and phosphorus in plasma. This is accompanied by an increase in the alkaline phosphatase enzyme (ALP) in plasma.
aCKNOWLEDGEMENTS
We would like to thank The College of Agriculture, AL-Qasim Green University for giving us this opportunity to express our science.
cONFLICT OF INTEREST
This research is a personal non-profit work and there is no
conflict of interest.
aUTHORS CONTRIBUTION
Both of Ammar Salahaldeen, Nihad Abdul-Lateef Aliare responsible for animal work and samples collection. Fadhil Rasul Abbas and Imad Abdul-Jabar, is responsible for data analysis, writing correction and proof reading.
REFERENCES





