Advances in Animal and Veterinary Sciences
Research Article
Ultrastructure, Morphological Differentiation and Pathological Changes of Ascaridia species in Pigeons
Mona Mohammed I. Abdel Rahman*, Hala M.N. Tolba, Heba M. Abdel-Ghany
Parasitology, Avian and Rabbit Medicine and Pathology, Departments Faculty Of Veterinary Medicine, Zagazig University, Egypt.
Abstract | Ascaridia species were the most common nematodes infecting pigeon. They included Ascaridia galli and Ascaridia columbae. The infected birds suffered from different clinical signs which varied from weight depression, retarded growth, decrease in egg production, gastrointestinal disturbance and intestinal blockage to high mortality rates, according to the severity of infections, therefore this study was aimed to determine their infection rates, the morphological differences by light and scanning electron microscopes (SEM), induced histopathological lesions in different visceral organs and the effect of green onion on A.galli cuticle in vivo. Our study reported 25% and 10% infection rate for A.galli and A. columbae, respectively. Microscopical examination revealed that the ultra structures of A. columbae resembled that of A. galli except for lip carrying two triangular teeth on its inner surface instead of two tooth-like projections in A. galli and absence of subannuli and cuticular vesicles instead of their presence in A. galli. In addition,the male caudal end carried ten and thirteen pairsof caudal papillae in A.galli and A.columbae, respectively. Exposure to green onion showed wrinkled cuticular surface in lips, loss some of labial amphids and the appearance of granulation tissue in the oral cavity of A. galli by SEM and showed cuticular damage by histopathological examination. Also,histopathology revealed hemorrhage, congested blood vessels, fatty degeneration, necrosis and leukocytic infiltrationin the examined liver, lungs, heart, intestine, brain and ovaries. Finally, our study recommends using finely chopped green onion as a food additive in case of infections with Ascaridia species because they had a significant anthelmintic effect on the cuticle and sensory papillae of A. galli.
Keywords | Allium cepa, Annuli, Columbae, Cuticle, Galli.
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | August 21, 2018; Accepted | November 01, 2018; Published | November 20, 2018
*Correspondence | Mona Mohammed I Abdel Rahman, Department of Parasitology, Faculty Of Veterinary Medicine, Zagazig University, Egypt; Email: mona_111_para@yahoo.com
Citation | Abdel Rahman MMIA, Tolba HMN, Abdel-Ghany HM (2019). Ultrastructure, morphological differentiation and pathological changes of Ascaridia speciesin pigeons. Adv. Anim. Vet. Sci. 7(2): 66-72.
DOI | http://dx.doi.org/10.17582/journal.aavs/2019/7.2.66.72
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2019 Abdel Rahman et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Domestic pigeons were important for human as a dietary protein source and for experimental studies as they were easy to breed, gained body weight rapidly and induced little noise (Natala et al., 2009). Parasitic diseases were the most important diseases affecting pigeons. They induced great losses in the poultry industry as they mightbe fatal, especially in squabs and heavy infested adults as well as they adversely affect on production and general health condition of birds (Weber 1979).
Ascaridia species were the most common nematodes infecting pigeon. They included Ascaridia galli and Ascaridia columbae. A.galli considered the biggest nematode infecting gastro intestinal tract of pigeon.The life cycle of A.galli is simple and direct where the infective eggs (egg contains second stage larva) hatch in the duodenum of bird. After hatching, free larvae penetrate the mucosa and cause hemorrhages. A few of the larvae might emerge to visceral organs, while the majority penetrates the intestinal mucosa and developed to adult worms. In addition, grasshoppers and earthworms acted as transport hosts for Ascaridia species and become infective to other birds, although no development of the larvae occurred (Soulsby, 1982). In A. columbae, larvae might emerge to the lung and liver, but moulting did not occur and remained undeveloped (Wehr and Hwang 1959; Wehr and Hwang 1964). Hygienic deficiency and favorable environmental conditions (high temperature and low humidity) will help in the recurrence of Ascaridia infections in pigeons.
The infected birds with Ascaridia suffered from different clinical signs which varied from weight depression, retarded growth, decrease in egg production, gastrointestinal disturbance and intestinal blockage to high mortality rates according to the severity of infections (Radfar et al., 2012).
Onion (Allium cepa) usage considered a type of traditional medicine that used by Indian folk to treat dysentery, fever, bronchitis, insect bites and skin diseases (Nadkarni, 1982). (Raharjo, 2014) had reported the Anthelmintic effect of onion (Allium cepa) on the A.galli in vitro. This effect was due to that onion contained tannin, saponin and flavonoid (Santosh et al., 2011). The anthelmintic effect of green onion in this study indicated by a decrease in the number of deaths, disappearance of diarrhea, improvement of appetite and general health conditions in infected pigeons.
The aim of the current research was to detect the infection rates of A.galli and A. columbae in domestic pigeons in sharkia province, Egypt and to recognize the morphological differences between them usinglight and scanning electron microscopes (SEM).This study also investigated the morphological changes in A.galli resulted from oral intake of chopped green onionas well as the response of the infected pigeonsto this as a preventive method for treatment. Finally, pathological lesions in visceral organs of infected pigeons were described.
MATERIALS AND METHODS
The total numbers of 100 domestic pigeons (Columba domestica) were admitted to the Department of Avian and Rabbit Medicine, Faculty of Veterinary Medicine, Zagazig University. They were collected from different markets and localities in Sharkia Province, Egypt with a history of eating finely chopped green onion as food additive by some of infected birds. The adult worms were collected during postmortem examination of their gastrointestinal tracts. This study was ethically approved by ZU-IACUC Committee, Zagazig University, Egypt (ZU-IACUC/2/F/6/2018).
After washing the collected worms with saline to remove any attached debris, they cleared in lactophenol, examined under light microscope and identified according to the keys described by (Ruff, 1984; Soulsby, 1982). Some worms were immediately fixed in 2.5% glutaraldehyde solution (PH 7.2) for 24 hours, then dehydrated in ascending graded ethanol, then they were coated with gold and examined with Quanta FEG250 scanning electron microscope, operated at 20 KV in National Research Center, Dokki, Egypt.
For histopathological examination, the collected specimens from intestine, liver, lungs, heart, ovary and adult worms were fixed in 10% buffered neutral formalin solution, dehydrated in gradual alcohol (70-100%), cleared in xylene and embedded in paraffin. Five micron thickness paraffin sections were prepared and stained with hematoxyline and eosin (HE) dyes and then examined microscopically (Suvarna et al., 2013).
RESULTS
Clinical Findings and Postmortem (Pm) Examination
The examined pigeons suffered from diarrhea, emaciation, anemia, off food and growth retardation. Also, some deaths were recorded in infected pigeons. During PM examination, we have noticed that the infected pigeons fed on the green onion had fewer numbers of live and some dead worms in the small intestine, but others that did not eat chopped green onion showed intestinal perforation, enteritis and there were more worms in their intestine and inside the abdominal cavities.
Ascaridia galli and Ascaridia columbae Infection Rates
During PM examination, we detected Ascaridia galli and Ascaridia columbae in small intestine of domestic pigeons.Their bodies appeared cylindical and creamy white-colored (Figure 1A & D).Their infection rates were 25% and 10%, respectively. A. galli reached 45-110 mm in length, but A. columbae reached 16-60 mm in length.
Light Microscopical Identification
The anterior end had three lips and a pair of lateral cephalic alae extending from the base of the lips to a short distance behind club-shaped oesophagus. The lips included one broad elliptical called mid-dorsal lip and two latero-vental lips. Each one had a broad base and tapered toward apex (Figure 1 B&E). The female caudal end had one pair of large caudal papillae (Figure 1C). Male caudal end carried ten pairs of caudal papillae, precloacal sucker and a pair of spicules. Precloacal sucker had an ill developed circular rim in A.galli, while it was chitinous-rimmed in A. columbae. In A.galli, the caudal papillae arranged as the following:precloacal (three pairs), cloacal (one pair), postcloacal (three pairs) and sub terminal (three pairs) (Figure 1G&H). While, in A. columbae: the caudal papillae included thirteen pairs (five precloacal and eight postcloacal). The spicules were sub equal with alae in A. columbae and without alae in A. galli (Figure 1H and Figure 2A, B&D). Their eggs were oval with smooth and thick shell. They measured 70x50 µ and 60x35µ in A. galli and A. columbae, respectively (Figure 1F & Figure 2C).
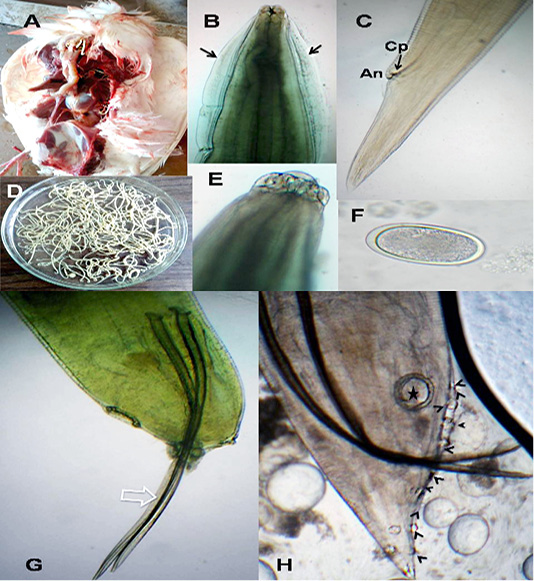
Figure 1: A. galli. A)Macrophoto of Ascaridia species in pigeon intestine; B) A. galli anterior end with cervical alae (arrows); C) Female tip tail with anus (An) and one pair of caudal papillae (Cp); D)Macrophoto of Ascaridia species; E) trilobed lips of A. galli; F) A. galli egg; G&H) Male caudal end of A. galli (large arrow: spicules, star: precloacal sucker, arrow heads: caudal papillae). (Digital camera)
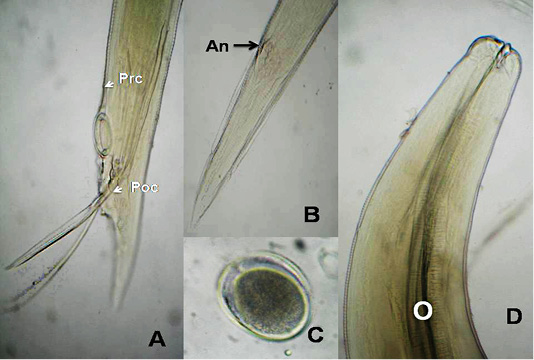
Figure 2: A. columbae, A) Male caudal end of (Prc: precloacal papillae, Poc: postcloacal papillae); B) Female tip tail with anus (An); C)egg; D) anterior end with club-shaped esophagus (O). (Digital camera)
Scanning Electron Microscopy (Sem)
In A. galli, each trilobed lip had a pair of eye-like labial papillae and two pairs of amphids. The amphids provided with minute submedial pores. Their inner surface carried two tooth-like projections. Cervical papillae located behind the base of the ventro-lateral lips (Figure 3A). Cuticle characterized by transversely striated annulations which subdivided by subannuli (Figure 3C). The ventral surface of male end provided with cuticular vesicles just behind the level of precloacal sucker and ten pairs of caudal papillae (Figure 3B). The ultra structures of A. columbae resembled the above described characters for A. galli except that the inner surface of each lip carried two triangular teeth (spoon-like), absence of subannuli and cuticular vesicles and thirteen pairs of caudal papillae (Figure 3D-G).
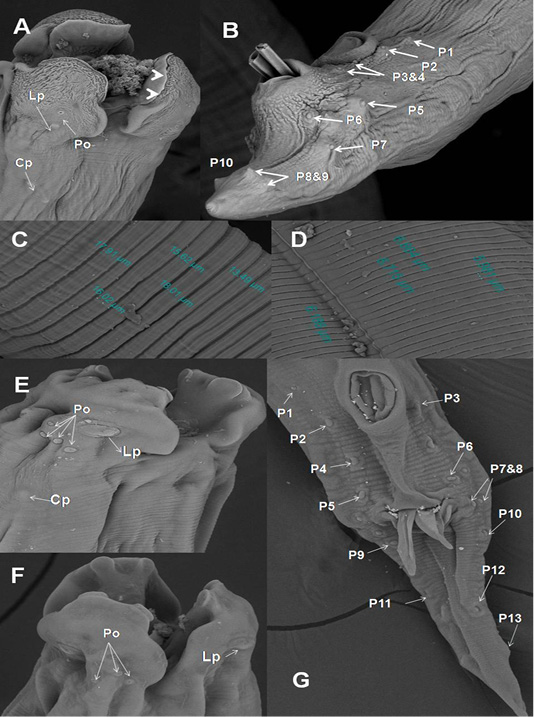
Figure 3: SEM of A. galli and A. columbae. A) Onion exposed A. galli anterior end with cuticular shrinkage of trilobed lips provided with inner 2 tooth-like projections (arrow heads). Also, oral cavity filled with granulation tissue and loss some of amphidial pores (2000x); B) Male caudal end of A. galli provided with cuticular vesicles and carried ten pairs of caudal papillae (800x); C)Wider A. galli cuticular transverse striation divided by subannuli (1600x);D) Wide A. columbae cuticular transverse striation (1600x); E&F) A. columbae trilobed lips carried triangular teeth, labial papillae, amphidial
Histopathological Examination
The liver showed congestion of central vein and hepatic sinusoids between hepatic cells together with fatty degeneration, which represented by multiple fat vacuoles inside the cytoplasm of hepatic cells. Some blood vessels showed mild thickening of its wall. Telangectiasis of hepatic sinusoids was seen (Figure 4A & 4B). There was coagulative necrosis with polymorphic cells infiltration among the hepatic cells (Figure 4C). Also, the larva of A. galli was found in liver (Figure 5G). Lung revealed follicular bronchiolitis represented by present numerous peribronchial lymphoid follicules with present some emphysematous alveoli (Figure 4D & 4E). There was thickening of interalveolar septa due to present extravasated erythrocytes beside hemosiderosis and leukocytic infiltrations (Figure 4F). Heart showed mild hyaline degeneration of the myocardial muscle beside congestion of blood vessels with hyaline thickening of its wall and perivascular edema (Figure 4G). There were extravasated erythrocytes between degenerated muscles and hemosidrosis (Figure 4H). Intestine revealed necrosis of the intestinal mucosa with desquamation of the lining epithelium inside the lumen. Focal aggregations of eosinophils were seen in the lamina propria among necrotic intestinal glands (Figure 5A & 5B). Brain showed hemorrhageand congestion of blood vessels beside degeneration of some neurons (Figure 5C). Ovary showed extravasated erythrocytes in the ovarian stroma beside congestion of some blood vessels (Figure 5D).
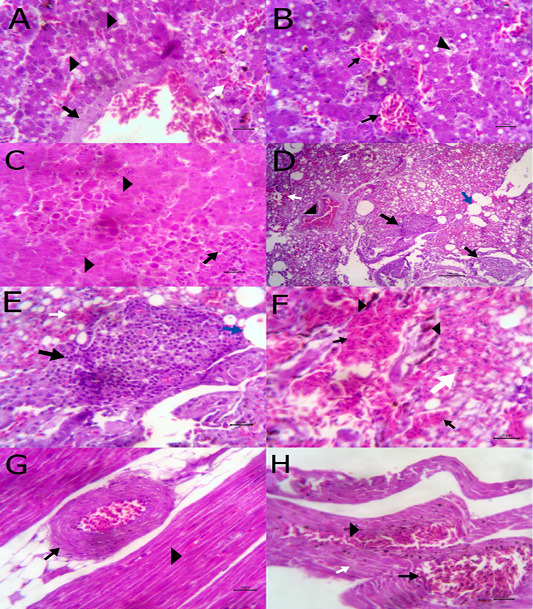
Figure 4: A) Liver showing congestion of central vein with mild thickening of its wall (arrow) and fatty degeneration (arrow head, bare=20); B) Liver showing telangectiasis of hepatic sinusoids (arrow) beside fatty degeneration (arrow head, bare=20); C) Liver showing coagulative necrosis among hepatic cells (arrow head) and aggregation of polymorphic inflammatory cells (arrow, bare=20); D) Lung showing follicular bronchitis(black arrow), hemorrhage among the alveoli (white arrow) and emphysematous alveoli (blue arrow, bare=100); E) Higher magnification of the
previous figure to show hyperplasia of lymphoid follicles (black arrow), hemorrhage (white arrow) and emphysematous alveoli (blue arrow, bare=20); F) Lung showing thickening of interalveolar septa by extravasated erythrocytes (arrow) beside hemosiderosis(arrow head)and leukocytic infiltrations (white arrow) bare=20; G) Heart showing mild hyaline degeneration of the myocardial muscle (arrow head) beside congestion of blood vessels with hyaline thickening of its wall and perivascular edema (arrow, bare=20); H) Heart showing extravasated erythrocytes (arrow) among degenerated muscle fibers (white arrow) with hemosiderosis (arrow head, bare=20).
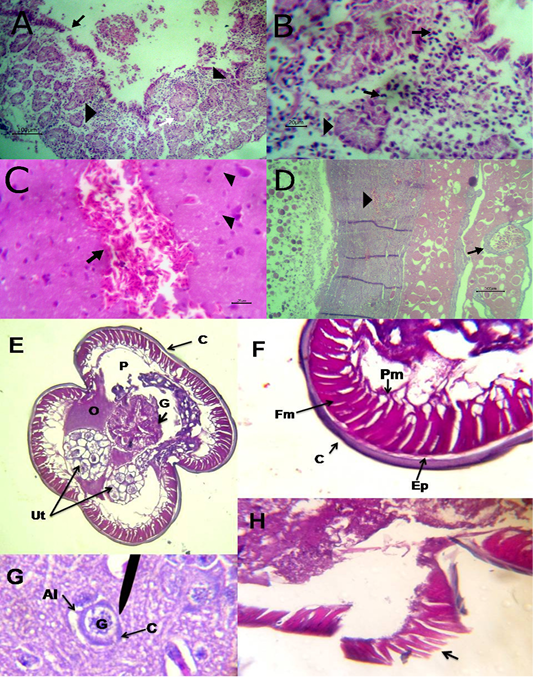
Figure 5: A) Intestine showing necrosis of intestinal mucosa with desqumation of lining epithelium inside the lumen (arrow) beside eosinophils aggregations in the lamina propria (arrow head) among necrotic gland (white arrow, bare=100); B) Higher magnification of the previous figure to show aggregations of eosinophils (arrow) among necrotic glands (arrow head, bare=20); C) Brain revealing extravasated erythrocytes (arrow) beside degeneration of some neurons (arrow head, bare=20);
The Observed Effect Of Finely Chopped Green Onion Taken Orally As Food Additive
SEM revealed that adult worms of A. galli obtained from pigeon treated orally with green onion showed wrinkled cuticular surface in lips, loss some of labial amphids and the appearance of granulation tissue in the oral cavity of worms (Figure 3A). In addition, when we followed the infected birds that ate green onion regularly, we noticed decrease in the number of deaths, the disappearance of diarrhea, improvement of appetite and general health condition of infected pigeons.
Histopathological cross section of A. galli adult worm obtained from birdsthat did not eat green onions as dietary supplement showed uterus, ovary and gut inside the peudocel covered by normally constituted body wall (Figure 5E). The body wall consisted of cuticle, epidermal layer, fibrillar and proplasmic muscle components (Figure 5F). However, the cross section of adult worm obtained from birds that took the chopped green onions as a dietary supplement showed damage of the cuticle and the epidermal layer (Figure 5H).
DISCUSSION
This study revealed 25% infection rate for A. galli. Nearly similar percentages were 22%, 38.94% and 32% by (Alkharigy et al., 2018) in Tripoli, Libya, (Abed et al., 2014) in Iraq and (Ghosh et al.,, 2014) in Bangladesh, respectively. Lower percentages were 18.87% by (Lhsony, 2014) in Libya, 16.7% by (Elmajdoub and Mshiheet, 2016) in Libya and 15.5% by (Msoffe et al., 2010) in Tanzania. The lowest percentages were recorded to be 5.9% in Iraq by (Al-Barwari and Saeed, 2012), 3.3% in Nigeria by (Adang et al.,, 2008) and 1.2% in Nigeria by (Natala et al., 2009).
In our study, the infection rate for A. columbae was 10%. Nearly similar rates 13.04% 12%, 11.3% and 8.4% were recorded by (Khezerpour and Naem, 2013) in Iran, (Nagwa et al., 2013) in Gharbia governorate, Egypt, (Adang et al., 2008) in Nigeria and (Bahrami et al., 2012) in Iran, respectively. Higher rates were 24.4% by (Al-Barwari and Saeed, 2012) in Iraq, 23.2% by (Ibrahim et al., 1995) in Ismailia, Egypt and 20.37% by (Borji et al., 2012) in Iran. The lower rates reported by (Laku et al., 2018) in Nigeria, (Djelmoudi et al., 2014) in Algeria, (El-Dakhly et al., 2016) in Beni-Suef province, Egypt and (Natala et al., 2009) in Nigeria to be 4.55%, 4.2%, 3% and 1.2%, respectively.
The variance in infection rates might be due to varied breed susceptibilities, climatic changes, presence of earth worm as a transport host, presence of contaminated premises with litter of other bird species, egg resistance to the used disinfectant and breeding system. (Lindquist, 1963) reported the transmission of A.columbae through crop milk from parents to squabs.
The morphological descriptions of lips, cuticle, female tip tail, number and arrangement of caudal papillae, precloacal sucker and spicules in male caudal end observed in this study were in agreement with (Ashour 1994; Banaja et al., 2013; Gomes et al., 2015; Kajerova et al., 2004; Ramadan and Abou Znada 1992; Teixeira et al., 2012) descriptions for A. galli. Also, the obtained morphological features for A. columbae were similar to those described by (Banaja et al., 2013; Ibrahim et al., 1995; Kajerova et al., 2004; Moudgil et al., 2017; Pinto et al., 1991; Wehr and Hwang 1964).
In our study, light microscopic examination revealed that the female of A.galli carried one pair of caudal papillae near its tip in spite of, (Kates and Colglazier 2011; Zhao et al., 2016) who couldn’t observe it. Also, (Lalchhandama, 2010) detected it only by using SEM.The cuticle striations of A. galli were subdivided by several subannuli. (Hassanain et al., 2009; Lalchhandama, 2010) explained that the subannuli resulted during larvae development into adult worms and increasing the length of the adult worm. (Lalchhandama, 2010) reported that the labial amphids acted as sensory organs which had chemotactic attraction force for larvae to complete their life cycle in suitable hosts.
Histopathological examination revealed hemorrhagic lesions in liver, lung, heart, brain, intestine and ovary occurred due to migration of larvae during the tissue phase of the life cycle. Also,this study showed the emergence of A. galli larvae in the liver of infected pigeons, while this did not detected in cases of infection with A. columbae. Thus, these results confirmed the statements of the previous authors where (Michel, 1974) noticed migration of A. galli larvae to liver and lung. In addition, (Wehr and Hwang, 1964) showed that the development of A. columbae larvae completed only in intestine and the aberrant larvae failed to develop in liver. Microscopical examination of liver showed congestion of blood vessels beside polymorphic cells infiltration with fatty degeneration. The lungs revealed thickening of interalveolar septadue to present extravasated erythrocytes beside hemosiderosis and leukocytic infiltrations. Heart showed mild degeneration of the myocardial muscle beside congestion of blood vessels with extravasated erythrocytes. Intestine revealed necrotic mucosa with focal aggregations of eosinophils in the lamina propria among necrotic intestinal glands. The brain showed hemorrhage and congestion of blood vessels beside degeneration of some neurons. The ovary showed extravasated erythrocytes in ovarian stroma beside congestion of some blood vessels. Similar finding were partially agreed with (Adang et al., 2010) who observed necrosis of liver, lung, heart, intestine and kidney with mononuclear cellular infiltration and congested blood vessels.
Mortality of pigeons infected with Ascaridia species occurred in heavy infection where their aggregation in the lower part of the intestine resulting in intestinal obstruction, while the observed inflammatory lesions in intestine was due to burrowing of worms which also recorded by (Ikeme, 1971; Soulsby, 1982).
The current study evaluated the effect of green onions taken orally with the pigeon’s food on the cuticle of A. galli in vivo. While, (Raharjo, 2014) studied the effect of onion bulb squeeze on lethal death time of A. galli in vitro. (Santosh et al., 2011) explained that the anthelmintic effect of onion resulted from tannin, saponin and flavonoid. In our study, the observed destructive effects of Allium cepa on the cuticular surface of lips andamphids prevent the worms from stabilizing themselves inside the intestines and drummed outside the infected pigeons. This was similar to the effect of ethanolic extract of Citrusmicrantha flowers observed by (Hassanain et al., 2009). Also, the destructive histopathological effect of Allium cepa on the cuticle and epidermal layer of A. galli was similar to that observed by (Balqis et al., 2017) where A. galli exposed ethanolic extract of Veitchiamerrillii betel nuts in vitro.
In conclusion, our study recommends using finely chopped green onion as a food additive because they had a significant anthelmintic effect on A.galli infecting pigeons. As the green onion considered a natural source for treatment and do not have a detrimental effect on the liver like chemical substances as mentioned by (Metwally, 2006) who reported that the onion oils improved the liver function and antioxidant status in infected cases with Schistosomamansoni. Therefore, using of finely chopped green onion mixed with pigeon’s food will help to overcome the drug resistance problem resulted from the repeated use of chemical drugs. Our research study will recommend studying the anthelmintic activity of green onion against other parasitic infections in vitro and in vivo in the future.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS CONTRIBUTION
Mona Mohammed I. Abdel Rahman designed and prepared the draft manuscript, Hala M.N. Tolpa provided guidance, technical support and edited the manuscript, Heba M. Abdel-Ghany carried on histopathological examination, provided guidance, technical support and edited manuscript.
References





