Advances in Animal and Veterinary Sciences
Research Article
In-Vitro Study the Antibacterial Activity of Bacteriocin against Stenotrophomonas Maltophilia and Evaluation its Synergism with some Antibiotics
Sarhan Rashid Sarhan*1, Orooba Mohammed S. Ibrahim2
*1College of Veterinary Medicine, Department of Physiology and Pharmacology, Wasit University, Wasit, Iraq; 2College of Veterinary Medicine, Baghdad University, Baghdad, Iraq.
Abstract | The core of the study is to screen the inhibitory effect of bacteriocin producing Lactobacillus acidophilus against isolated S. maltophilia and examine whether the combination of bacteriocin with antibiotics showed synergistic or antagonistic interaction. The majority of isolates were resistant to two antibiotics and the isolate coded S19 was resistant to the three antibiotics used, which is consider as multi-drugs resistant isolate and used for the synergy test. Initially, minimum inhibitory concentrations (MICs) of Ceftazidime (CAZ), Imipenem (IMP) and Minocycline (MIN) were determined using exponentially growing cultures of S. maltophilia. The MICs of antimicrobial were 64, 32 and 128μg/ml, respectively. When antimicrobial and bacteriocin combined in the checkerboard microtiter test, a synergistic effect was observed and the MICs decreased dramatically. All of the three antimicrobials with bacteiocin combinations showed synergy against the resistant S. maltophilia isolate (S19).A combination of Imipenem plus bacteiocin showed the highest synergistic effect among the combinations. FIC index of a combination of Imipenem, Ceftazidime and Minocyclin with bacteriocin, were 0.249, 0.093 and 0.312 for S. maltophilia isolate respectively. Antagonistic activity was not found in any of the combinations. In conclusion in the presence of bacteriocin, a lower Ceftazidime, Imipenem and Monocycline concentrations were needed to fully inhibit S. maltophilia .Checkerboard assay was appropriate method to calculate the MIC and combination testing of the bacteriocin and antibiotic as well as the fractional inhibitory concentration index (FICI) was applicable to aid in an interpretation of the combination testing results.
Keywords | Bacteriocin producing LAB, Bacterial resistance, Stenotrophomonas maltophilia, Checkerboard assay.
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | September 08, 2018; Accepted | October 14, 2018; Published | November 02, 2018
*Correspondence | Sarhan Rashid Sarhan, College of Veterinary Medicine, Department of Physiology and Pharmacology, Wasit University, Wasit, Iraq; Email: srashid@uowasit.edu.iq
Citation | Sarhan SR, Ibrahim OMS (2018). In-vitro study the antibacterial activity of bacteriocin against stenotrophomonas maltophilia and evaluation its synergism with some antibiotics. Adv. Anim. Vet. Sci. 6(12): 556-568.
DOI | http://dx.doi.org/10.17582/journal.aavs/2018/6.12.556.568
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2018 Sarhan and Ibrahim. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Stenotrophomonas maltophilia has emerged as an opportunistic nosocomial pathogen of increasing importance. As it is intrinsically resistant to a broad spectrum of antimicrobial agents, the treatment of infections due to S. maltophilia is usually problematic in clinical practice, leading to a high frequency of treatment failure and mortality (Denton and Kerr, 1998; Gales et al., 2001). The antimicrobial resistance of S. maltophilia is attributed to the reduction in outer membrane permeability, expression of efflux pumps, or production of multiple beta-lactamases (Zhang et al., 2000; Li et al., 2002). The expression of two or more of these resistance mechanisms together usually results in the development of multidrug resistance (MDR), a condition that may necessitate the use of antimicrobial agents in combination. Antimicrobial susceptibility testing of S. maltophilia isolates presents some problems, and susceptibility testing guidelines have not yet been fully established for this microorganism. The US Clinical Laboratory and Standards Institute (CLSI) recommends the use of the broth or agar dilution method and disc diffusion method for testing minocycline, levofloxacin, and trimethoprim/ sulfamethoxazole (TMP–SMX); and the broth or agar dilution method for testing ticarcillin/clavulanic acid, ceftazidime, and chloramphenicol (CLSI, 2018).The recent increase in the number of cases of antibiotic resistance has encouraged scientists to reassess alternative therapeutic options (Michael et al., 2014; Holmes et al., 2016) In general, however, the widespread discovery of novel antibiotics remains largely uncommon (Cooper and Shlaes, 2011).
Bacteriocin is one of the effective option in treatment of many bacterial infection as alternative therapeutics option to compensate the shortage of novel antibiotics. Bacteriocin is a peptides synthesized in bacterial ribosome toxic to other bacteria and it is reveal a narrow spectra of activity which is targeting a group of related bacteria, while other show wide spectrum effective against other species and genera (Cotter et al., 2013).
Bacteriocins produced by Gram-positive bacteria are of great interest for researchers because they are produced by useful lactic acid bacteria (LAB) in addition to generally having a wider inhibition spectrum than bacteriocins from Gram-negative bacteria. Bacteriocins produced by LAB are also generally regarded as safe (GRAS), since they can be found or used in fermented food and feed products like cheese and yoghurt in addition to being non-toxic to eukaryotic cells (Nes et al., 2007). LAB produces various compounds such as organic acids, diacetyl, hydrogen peroxide, and bacteriocin or bactericidal proteins during lactic fermentations. Bacteriocins are antimicrobial proteinaceous compounds that are inhibitory towards sensitive strains and are produced by both Gram-positive and Gram negative bacteria (Padmanabha et al., 2006).
However, possibly a good option to combine the bacteriocins with other antibiotics. A synergy between bacteriocins and antimicrobial may potentiate each other killing activity, thus reducing the emergence of resistance to either antibiotics and bacteriocins.
Furthermore, combinations of bacteriocins with antibiotics can decrease the concentration of antibiotics required to kill a target pathogen, thereby diminishing the likelihood of adverse side effects associated with the antibiotic. An example of adverse effects of polymyxin group of antibiotics is the nephrotoxicity (Mendes et al., 2009; Abdelraouf et al., 2012). One of the advantages of synergistic combinations of bacteriocins with antibiotics are decreases the funding associated with the synthesis and introducing of the more costly antibiotics. As well as, efficacious synergistic combinations of antibiotics and bacteriocins can extend the spectra of antibiotics, which may be beneficial in treating bacterial infections of unknown etiology. There are many methods to assessing antibacterial synergy in vitro. Examples of such tests include the broth-based checkerboard assay, as well as agar-based screens such as E-tests (bioMérieux) to evaluate synergy (Sopirala et al., 2010; Soltani et al., 2012). Therefore, the present study set out to screen the synergism interaction between some antibiotics and bacteriocin producing Lactobacillus acidophilus against isolated S. maltophilia.
MATERIALS AND METHODS
Isolation and Identification of Stenotrophomonas Maltophilia
A total of a 75 swab samples were collected from different sites of infection in dogs during Dec 2017 to Feb 2018. Samples were obtained from different locations in Wasit province. Samples were diluted in saline and swabbed on blood-supplemented Mueller–Hinton agar plates MHA (Hi-media, India). Specimens were promptly took to the laboratory and processed. Standard methods for isolation and identification of S. maltophilia were used.
Lactobacillus acidophilus
L. acidophilus was obtained from central public health lab. In Wasit province. The bacteria was re-cultured on Do Man Rogosa sharpe agar (MRS) (Himedia, India) incubated anaerobically with gas generating kit (5-10% CO2 of atmosphere) at 37 Co for 24 hr. (MacFaddin, 2000). Overnight culture was centrifuged at 10000 xg for 15 min. The supernatant was collected and passed through 0.2 um sterile syringe filter. The supernatant broth collected were used for antibacterial study against S. maltophilia (Astha et al., 2012). For elimination of the antimicrobial effect of organic acids the supernatant fluid adjusted to pH 6.5 with NaOH (1 N) to rule out acid inhibition. Also the inhibitory action of hydrogen peroxide was eliminated by the addition of a sterile solution of catalase (Fluka, Germany) (300 U/ml) at 25°C for 30 min (Ammor et al., 2006).
Susceptibilty of the S. Maltophilia to Antibiotics
Minimum Inhibitory Concentration (MIC) was determined by using broth dilution assay method (CLSI, 2018).In the tube dilution assay, standard bacterial suspension (S19) (3.5×107 cell/ml) was added to tubes containing 9 ml Cation adjusted Mueller–Hinton broth MHB (Hi-media, India) and different concentrations of Ceftazidime, Imipenem and Minocycline, Table (1) (0.5, 1, 2, 4, 8, 16, 32, 64, 128 and 256µg/ml), (0.125, 0.25, 0.5, 1, 2, 4, 8, 16, 32 and 64µg/ml) and (2, 4, 8, 16, 32, 64, 128, 256, 512 and 1024µg/ml) were prepared respectively. Two tubes containing antibacterial and nutrient broth served as negative control and positive control, respectively. After 24 h incubation at 37C°, the tubes were examined for growth. The MIC of antibiotics was taken as the lowest concentration that showed no growth. Multiple drugs resistant isolate that showed resistant to the all three antibiotics was used for the further experiments.
Table 1: Antibiotics used in this study
| Antibiotics/Chemicals | Company | Origin |
| Ceftazidime | GlaxoSmithKline Pharmaceuticals Ltd | England |
| Imipenem | Ranbaxy Laboratories Ltd. | India |
| Minocycline | Melinta Therapeutics |
US |
Susceptibilty of the S. Maltophilia to Bacteriocin
Determination of MIC of bacteriocin by using broth microdilution method. In this method, round-bottom wells of 96-well plates with a volume of 300 μl were used. Different concentrations of cell free supernatants were prepared by serial dilutions of 200 μL with 2 fold dilutions (300, 150, 75, 37.5, 18.75, 4.68, 2.34 and 1.17 μg/mL) using cation adjusted MHB (Himedia, India) then 100 μl of the bacterial suspension coded (S19) were added to each well. The final concentration of bacteria in each well was adjusted to 0.5 McFarland standard tube. Two wells containing microbial growth and media broth served as positive control and negative control, respectively. The plates were incubated at 37°C for 24. MIC values were detected by ELISA reader. (Dasari et al., 2014).
% of reduction in growth = OD value of control well – test well / OD value of control well × 100
Synergism Test of Bactriocin Combination with Antibiotics
The efficiency of double combination of Bacteriocins with Ceftazidime, Imipenem and Minocycline against the clinical resistant isolate of S. maltophilia (S19) was assessed by the checkerboard method. The synergy was evaluated by calculation of the Fraction Inhibitory Index (Σ FIC) as follow:
The interaction is defined as synergistic if the FIC index is ≤0.5; indifferent, if the FIC index is >0.5 and ≤4; and antagonistic if the FIC index is >4 (Turgis et al., 2016). To a standard 96-well round-bottomed microtitre plate, 50 μL of the bacteriocin and antimicrobial solutions were pipetted into each well, so that each row and column contained a ½ MIC, ¼ MIC, 1/8 MIC, 1/16 MIC, 1/32 MIC, 1/64 MIC and 1/128 MIC of bacteriocin and one antimicrobial agent as explained in Figures (1,2,3), Then inoculate each well with 100 μL volume of resistant isolate of S. maltophilia, equivalent to 0.5 MacFarland suspension was pipetted (Andrews, 2001). All microtitre plates were incubated at 37CO for 24 h. At the end of the incubation period, each well was observed for optical density. The lowest concentration that did not show turbidity was taken as the MIC. The fractional inhibitory concentrations (FIC) were deter
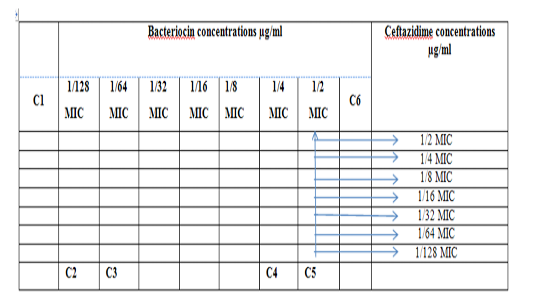
Figure 1: Diagram of the checkerboard assay shows wells concentrations of Bacteriocin and Ceftazidime. C1 = broth alone (no bacterial inoculum), C2 = broth+ indicator + org, C3 =broth+indicator, C4 = Ceftazidime alone, C5 = D.W. alone and C6 = Bacteriocin alone.

Figure 2: Diagram of the checkerboard assay shows wells concentrations of Bacteriocin and Imipenem. C1 = broth alone (no bacterial inoculum), C2 = broth+ indicator + org, C3 =broth +indicator, C4 = Imipenem alone, C5 = D.W. alone and C6 = Bacteriocin alone.
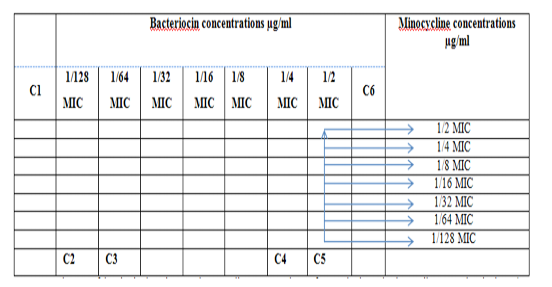
Figure 3: Diagram of the checkerboard assay shows wells concentrations of Bacteriocin and Minocycline. C1 = broth alone (no bacterial inoculum), C2 = broth+ indicator + org, C3 =broth +indicator, C4 = Minocycline alone, C5 = D.W. alone and C6 = Bacteriocin alone.
mined based on the method of (isobolographic analysis) described by (Tallarida, 2006). Six-fold replication of all checkerboard assays check for consistency and enabling the results to be provided as mean values. significant differences between data sets for each combination of bacteriocin were determined using the Wilcoxon Mann–Whitney test, and results that showed (P≤ 0.05) were considered as significant.
Isobolographic Analysis
For isobolographic analysis of the interaction of bacteriocin with Ceftazidime, Imipenem and Minocycline, the checkerboard data were analyzed by non-weighted, nonlinear regression analysis by using fraction concentration of each drug would be represented on the x and y axis and a straight line showing an inversely proportional relationship represents a purely additive effect between the drug (Tallarida, 2006).
Statistical Analysis
Data were analyzed statistically using the Microsoft Program SAS (Statistical Analysis System - version 9.1). Statistical analysis of data was performed on the basis of One-Way Analysis of Variance (ANOVA) using a significant level of P<0.05. (SAS, 2012)
RESULTS
Isolation and Identification of S. Maltophilia
According to standard laboratory examinations such cultural characteristics and biochemical tests indicated that a total of 75 samples were collected from dogs, 39 samples, showed positive results for the existence of S.maltophilia. Results summarized in Table (2), (3) and (4).
Table 2: The prevalence of S.maltophilia in nasal swab sample isolated from dogs.
| Isolates | No. of Samples | Percentage % |
| S. maltophilia | 39 | 52 |
| Others | 36 | 48 |
| Total | 75 |
100 |
Table 3: Cultural Characteristics tests used to identify S.maltophilia
| Cultural Characteristics | Results |
| Gram stain | G -ve bacilli |
| MacConkey Agar | Rounded, smooth convex colonies goldin yellow in color. |
| Chocolate Agar | golden yellow to brown colonies |
| VIA Agar | golden yellow to orange colonies |
| Blood Agar | Brownish discoloration of the medium around confluent growth |
| CLED Agar | Rounded, smooth convex colonies yellowish to bluish in color |
Susceptibilty of The S. Maltophilia to Antibiotics And Bactriocin
MIC of bacteriocin and antibiotics and the percentages of resistant S. maltophilia are shown in Tables (5) and (6).
Antimicrobial Combinations in Checkboard Assay
Combination of Bacteriocin with the three antimicrobials with showed synergy effect against the resistant S. maltophilia isolate coded (S19).A combination of Imipenemplus bacteiocin showed the highest synergistic effect among the combinations comparing to other antibiotics. The fractional inhibitory concentration (FIC) index of a combination of Imipenem, Ceftazidime and Minocyclin with bacteriocin, were 0.249, 0.093 and 0.312 for S. maltophilia isolate respectively. Antagonistic activity was not found in any of the combinations, results summarized in Table (7), Figures (4), (5) and (6).
Initially, minimum inhibitory concentrations (MICs) of Ceftazidime, Imipenem and Monocycline were determined using exponentially growing cultures of S. maltophilia. Our finding showed that the MICs of antimicrobial were 64, 32 and 128μg/ml, respectively.
When antimicrobial and bacteriocin combined in the checkerboard microtiter test, a synergistic effect was observed and the MICs decreased dramatically, Table (8). In the presence of low concentrations of bacteriocin, a lower concentrations of Ceftazidime, Imipenemand Monocycline were needed to fully inhibit S. maltophilia growth as illustrated in Figures (7), (8) and (9) represented the isobolography
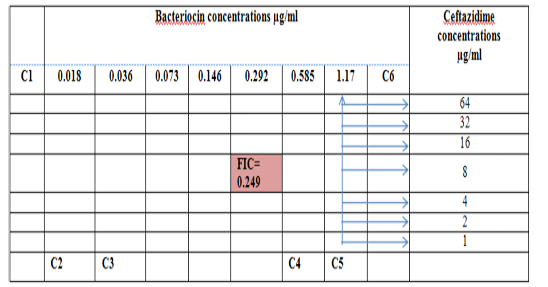
Figure 4: Diagram of the checkerboard assay shows well concentration of Bacteriocin and Ceftazidime. C1 = broth alone (no bacterial inoculum), C2 = broth+ indicator + org, C3 =broth +indicator, C4 = Ceftazidime alone, C5 = D.W. alone and C6 = Bacteriocin alone.
DISCUSSION
In veterinary medicine S. maltophilia is considered to be a coloniser. In domestic animals, there are only a few reports
Table 4: Biochemical tests used to identify S. maltophilia
| Biochemical tests | TSI | Maltose oxidation | Glucose | Catalase test | Lysine decarboxylation | Oxidase test |
| Results | K/K | +ve | +ve | +ve | +ve |
-ve |
Table 5: Antimicrobial activity of Antibiotics and Bacteriocin against isolated (S19) from dogs
| Bacteria |
S. maltophilia (n= 39) |
|||
| MIC µg/ml | S n(%) | I n(%) | R n(%) | |
| Antibiotics / Bacteriocin | ||||
| Ceftazidime | 64 | 5 (12.8%) | 6 (15.38%) | 28 (71.79%) |
| Imipenem | 32 | 23 (58.97%) | - | 16 (41.02%) |
| Minocycline | 128 | 12 (30.76%) | 8 (20.51%) | 19 (48.71%) |
| Bacteriocin | 2.34 | - | - |
- |
S: sensitive, I: intermediate, R: resistance
Table 6: Susceptibility percentage of S. maltophilia isolates to the antibiotics.
| Antibiotics | Susceptibility Percentage (%) | Ch-square | |||||
|
S.maltophilia Code no. |
ceftazidime | Imipenem | minocycline | % of S* | % of I* | % of R* | |
| S1 | R | S | I | 46.34 | 35.54 | 18.12 | 9.12* |
| S2 | R | S | I | 44.12 | 32.3 | 23.54 | 9.33* |
| S3 | R | S | S | 20.23 | 00.00 | 79.77 | 10.11* |
| S4 | R | S | S | 23.13 | 00.00 | 76.87 | 10.54* |
| S5 | R | R | I | 00.00 | 30.12 | 69.88 | 9.88* |
| S6 | S | R | I | 33.56 | 31.23 | 35.21 |
8.22* |
| S7 | S | R | I | 31.31 | 32.78 | 35.91 | 9.14* |
| S8 | S | S | R | 68.34 | 00.00 | 31.66 | 8.88* |
| S9 | R | S | R | 21.88 | 00.00 | 78.12 | 10.84* |
| S10 | S | R | R | 18.24 | 00.00 | 81.76 | 12.38* |
| S11 | R | S | R | 20.11 | 00.00 | 79.89 | 10.12* |
| S12 | R | R | S | 17.31 | 00.00 | 82.69 | 13.94* |
| S13 | S | S | R | 78.77 | 00.00 | 21. 23 | 9.16* |
| S14 | R | R | S | 15.41 | 00.00 | 84.59 | 13.81* |
| S15 | R | S | S | 74.44 | 00.00 | 25.56 | 10.18* |
| S16 | R | S | S | 85.44 | 00.00 | 14.56 | 9.65* |
| S17 | I | S | S | 75.89 | 24.11 | 00.00 | 8.77* |
| S18 | R | S | S | 81.63 | 00.00 | 18.37 | 11.55* |
| S19 | R | R | R | 00.00 | 00.00 | 100 | 15.00 |
| S20 | R | S | S | 77.34 | 00.00 | 22.66 | 11.10* |
| S21 | R | S | R | 37.98 | 00.00 | 62.02 | 8.54* |
| S22 | R | R | S | 33.56 | 00.00 | 66.44 | 9.25* |
| S23 | R | R | I | 00.00 | 37.65 | 62.35 | 9.72* |
| S24 | R | S | R | 28.65 | 00.00 | 71.35 | 10.77* |
| S25 | R | S | R | 12.65 | 00.00 | 87.35 | 12.31* |
| S26 | R | S | R | 18.75 | 00.00 | 81.25 |
12.45* |
| S27 | R | S | I | 36.15 | 22.73 | 41.12 | 8.61* |
| S28 | R | S | R | 28.45 | 00.00 | 71.55 | 9.41* |
| S29 | R | S | S | 79.13 | 00.00 | 20.87 | 10.38* |
| S30 | R | S | R | 23.64 | 00.00 | 76.36 | 11.82* |
| S31 | R | R | I | 00.00 | 40.46 | 59.54 | 8.91* |
| S32 | R | S | R | 37.88 | 00.00 | 62.12 | 9.18 |
| S33 | R | S | R | 32.83 | 00.00 | 67.17 | 9.19* |
| S34 | R | R | S | 39.32 | 00.00 | 60.68 | 8.85* |
| S35 | I | R | R | 00.00 | 36.63 | 63.37 | 9.63* |
| S36 | I | R | R | 00.00 | 33.98 | 66.02 | 10.23* |
| S37 | I | R | R | 00.00 | 41.52 | 58.48 | 10.76* |
| S38 | I | R | R | 00.00 | 37.42 | 62.58 | 8.18* |
| S39 | I | R | R | 00.00 | 31.94 | 68.06 | 11.91* |
| % of S* | 12.8% | 58.97% | 30.76% | *(p≤0.05) | |||
| % of I* | 15.38% | 00.00 | 20.51% | ||||
| % of R* | 71.79% | 41.02% | 48.71% | ||||
| Ch-square | 12.33* | 9.23* |
8.64* |
||||
S: sensitive, I: intermediate, R: resistance
Table 7: Effects of antimicrobials combinations with bacteriocin against clinical isolates S. maltophilia (S19).
|
Antimicrobial combinations WITH Bactriocin
|
|||
| Ceftazidime + Bacteriocin | Imipenem + Bacteriocin | Monocycline + Bacteriocin | |
| FIC* | 0.249 | 0.093 | 0.312 |
FIC: Fractional Inhibitory Concentration (FIC).
Table 8: Antimicrobial combinations in which resistance phenotype is changed to susceptibility.
| Combination of antimicrobial agents with Bacteriocin | MIC variation µg/ml | |
| Bacteriocin | Antibiotics | |
| Ceftazidime + Bacteriocin | 2.34 0.292 | 648 |
|
Imipenem + Bacteriocin |
2.34 0.073 | 32 2 |
| Monocycline + Bacteriocin | 2.34 0.585 |
128 16 |
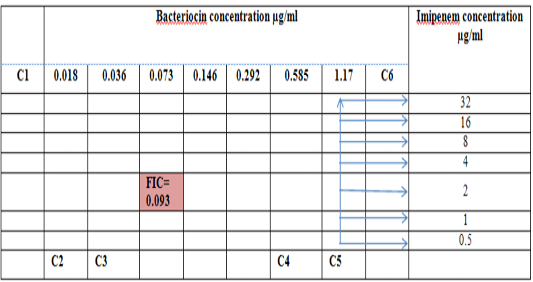
Figure 5: Diagram of the checkerboard assay shows well concentration of Bacteriocin and Imipenem. C1 = broth alone (no bacterial inoculum), C2 = broth+ indicator + org, C3 =broth +indicator, C4 = Imipenem alone, C5 = D.W. alone and C6 = Bacteriocin alone.
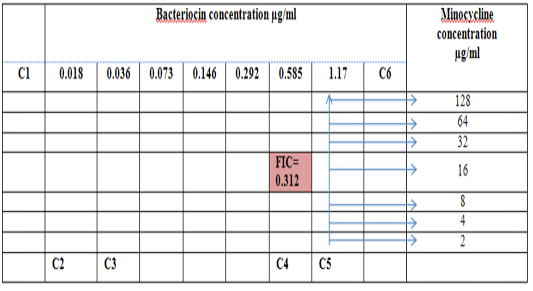
Figure 6: Diagram of the checkerboard assay shows well concentration of Bacteriocin and Minocycline. C1 = broth alone (no bacterial inoculant), C2 = broth+ indicator + org, C3 =broth +indicator, C4 = Minocycline alone, C5 = D.W. alone and C6 = Bacteriocin alone.

Figure 7: Minimum inhibitory concentration of Ceftazidime and Bacteriocin before and after combination by Isobolographic analysis.
dealing explicitly with S. maltophilia infection. These have detailed the isolation of the bacterium from the airways of patients with chronic respiratory disease (dog, cat, horse)
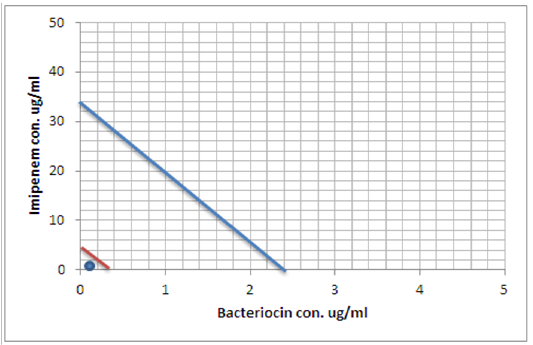
Figure 8: Minimum inhibitory concentration of Imipenem and Bacteriocin before and after combination by Isobolographic analysis.
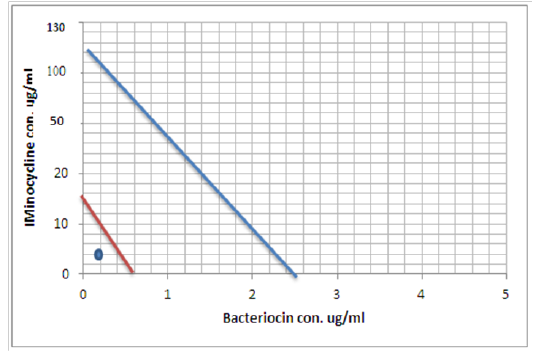
Figure 9: Minimum inhibitory concentration of Minocycline and Bacteriocin before and after combination by Isobolographic analysis
(Albini et al., 2009; Winther et al., 2010). A case report demonstrated that a urinary bladder biopsy specimen culture revealed a positive S. maltophilia (Kralova-Kovarikova et al., 2012). S. maltophilia is a ubiquitous organism commonly isolated from water, soil, and sewage and also from nosocomial environments. This organism can be the cause of respiratory, urinary, and bloodstream infections in hospitalized patients, especially in those who are immumocompromised or are in intensive care units and particularly in those patients who are catheterized or are receiving mechanical ventilation (Nicodemo et al., 2004). It can also cause infections in animals such as respiratory infections with chronic coughing in horses, canines, and bovines (Albini et al., 2009; Ohnishi et al., 2012; Winther et al., 2010). Julve et al. (1998) found that S.maltophilia may cause a wide range of clinical syndromes and is more likely to cause infection or colonization in patients who have underlying disease. To improve the isolation of S. maltophilia from swab samples, VIA agar was more selective to S. maltophilia and preventing the growth of unwanted bacteria from contaminated specimens. Culture media have been developed to differentiate between the bacterial species present in mixed culture samples (e.g., colony color differences between P. aeruginosa and S. maltophilia show the difference in their metabolic capabilities). Distinguishing S. maltophilia from P. aeruginosa can depend on, acid production from maltose and not produce acid from glucose in case of S. maltophilia, but in case of P. aeruginosa produces acid from glucose and does not utilize maltose or lactose to a large extent. The S. maltophilia colonies appear yellow and blue on BTB-containing medium that has both maltose and glucose, as opposed to P. aeruginosa colonies, that have a blue color on BTB medium having maltose and a yellowish color with glucose medium as in, this results were in agreement with (Denton, et al., 2000; Kataoka, 2003; Pinot et al., 2011). In which that the VIA medium was particularly useful for the detection of low colony counts (102 to 106 CFU/ml). According to the Clinical and Laboratory Standards Institute that provides guidelines for the testing of antimicrobials against S. maltophilia utilizing the dilution method (CLSI, 2011 to CLSI, 2018). S. maltophilia infections are difficult to treat due high levels of intrinsic resistance and also acquired resistances possibly due to overuse of broad-spectrum β-lactam antimicrobials (Gales et al., 2001). In current work, we study the suscptibilty of S. maltophilia isolates to different antibiotics and bacteriocin. S. maltophilia isolates exhibit differences in antimicrobial susceptibility to antibiotics such as ceftazidime (CAZ) , imipemen (IPM) and minocycline (MIN). In our study ceftazidime MIC was 64 μg/ml, while the MIC of imipenem was 32 μg/ml and the MIC of minocycline was 128 μg/ml. The resistance rate of S. maltophilia toward CAZ, IPM and MIN were 71.79%, 41.02% and 48.71%, respectively. The majority of isolates were resistant to two antibiotics and the isolate coded S19 was resistant to the three antibiotics used, which is consider as multi-drugs resistant isolate and used for the synergy test. Our results showed almost similar to what is Sun et al., 2016 found in his work, the resistant rate of S. maltophilia to CAZ was 60.8% (Erlin et al., 2016). Another study by Hejnary et al., demonstrated that the resistant rate toward CAZ is also high which is reached 44.4%. (Hejnary et al., 2010).
Mackenzie et al., studied the antibacterial activity of some β-lactam antibiotics such as imipenem (IMP) and non β-lactam antibiotics such as minocycline (MIN), they found that S. maltophilia resistant to IMP and the MIC range was 64-128 ug/ml higher what we found in our work. While the MIC of MIN range was 1-16 ug/ml. (Mackenzie et al., 2004).
Stenotrophomonas one of the clinically important nosocomial pathogen has significantly increased largely over the last twenty years. S. maltophilia can cause bacteraemia, endocarditis, pneumonia, meningitis, infections of bones and joints, urinary tract, soft tissues, and wounds (Falagas et al. 2009). It can also cause infections in animals such as respiratory infections with chronic coughing in horses, canines, and bovines (Albini et al., 2009; Ohnishi et al., 2012; Winther et al., 2010). One of the major features of S. maltophilia is the presence of numerous antibiotic resistance coding genes and efflux pump operons that confer frequent Multi-Drug Resistant (MDR) phenotypes among both clinical and environmental isolates (Youenou et al., 2015). Its genome is also characterized by the presence of several genes involved in virulence such as hemolysin, protease, phospholipase genes, and the smf1-operon which permits biofilm formation (Adamek et al., 2014).
The drug resistance mechanisms are acquired by the horizontal transfer of antibiotic resistance through transposons, integrons, plasmids. integron-like elements, insertion sequence common region (ISCR) elements, and biofilms. (Liaw et al., 2010; Hu L et al., 2011). There are limited antimicrobial options for infections due to S. maltophilia because of its extensive resistance to most antibiotics, including β-lactam antibiotics, cephalosporins, macrolides, aminoglycosides, and carbapenems. Interpretive breakpoints for susceptibility are available only for ticarcillin/clavulanate, ceftazidime, minocycline, levofloxacin, trimethoprim/sulfamethoxazole (TMP/SMX), and chloramphenicol (CLSI, 2018). TMP/SMX is recognized as the drug of choice (Wang et al., 2014). Resistance rates vary geographically but are generally less than 10% (Chung et al., 2013). However, high and various rates of resistance to TMP/SMX have been reported in patients with cancer (Vartivarian et al., 1994; Micozzi et al., 2000), a BSAC surveillance study (Livermore et al., 2008), and three large-scale multi-national studies (Sader et al., 2005; Farrell et al., 2014) ranged from90 to100%. Ceftazidimeandticarcillin/clavulanate used to be the most effective among b-lactam drugs against S.maltophilia. However, recent studies have demonstrated resistance rates of more than 30% and a trend in decreasing susceptibility with ceftazidime (47–75% during 1997–1999 to 30.5–36.8% during 2009–2012) (Gales et al., 2001; Sader et al., 2014).During 1997–1998,the ratesof susceptibility of S.maltophilia toticarcillin/clavulanate combination was 71–90%, while during 2003–2008 decreased to 27–46.1%.
Nowadays there are too many researches for discovering a new antimicrobials with potential activity and with less opportunity to induce antimicrobial resistance (Bush et al., 2011). Attractive substances from which novel antibiotics may be developed are the bacteriocins, a group of bacterially produced compounds used to fight other bacteria (Cotter et al., 2013). Bacteriocins behave as amphipathic, and kill microbes through the interaction of a positive charge of bacteriocin with negatively charged microbial membrane structures such as Lipopolysaccharide and Lipoteichoic Acid. These mechanisms are more difficult to evade by developing resistance, compared to metabolic enzymes which usually are targets for conventional antibiotics. (Hazem et al., 2016).
The present study correlates with many other studies which have revealed that Lactobacillus could produce organic acids, hydrogen peroxide and bacteriocins (Soccol et al., 2010). Our finding revealed that the supernatant extracted was contains 3mg/mL of total protein in a 100 mL of production medium. The extracted material was examine for their antibacterial activity, using a microtiter assay method. The compound was serially diluted in a two-fold dilution to obtain minimal inhibitory concentration (MIC) for the inhibition of bacterial growth. Of the tested concentrations, 2.34 μg/mL was found to be the MIC for resistant isolate. A study conducted by Dasari et al., demonstrated that Lactobacillus producing antimicrobial compounds inhibits the growth of cervical pathogens, 140 μg/mL was found to be the MIC for both the E. coli and Bacillus. (Dasari et al., 2014).
Lactobacilli are naturally present or deliberately added as starter cultures in unpasteurized milk and dairy products such as cheeses, yogurts and fermented milks (Coeuret et al., 2004).Yoghurt is a common product in which probiotic bacteria can be delivered to the human lower gut is made by fermentation of milk with the starter cultures Lactobacillusdelbrueckii subspecies bulgaricus and Streptococcus thermophilus (Hamilton-Miller, 2004). LAB produces various compounds such as organic acids, diacetyl, hydrogen peroxide, and bacteriocin or bactericidal proteins during lactic fermentations. Bacteriocins producing lactic acid bacterial isolates “generally recognized as safe (GRAS)”. Bacteriocins are antimicrobial proteinaceous compounds that are inhibitory towards sensitive strains and are produced by both Gram-positive and Gram negative bacteria (Padmanabha et al., 2006). Bacteriocins are often confused in literature with antibiotics or other types of peptides with antimicrobial activity. This confusion is the most important legal standpoint to limit their use in industrial applications (Balciunas et al., 2013). When they are compared, bacteriocins have ribosomally synthesized nature, while antibiotics are produced by multi-enzyme complexes. Many times bacteriocins show bactericidal or bacteriostatic effects on a narrow spectrum of bacteria, but traditional antibiotics have a broader spectrum. Moreover, most bacteriocins are more effective against their target bacteria than antibiotics at lower concentrations. There are also slight differences between bacteriocins and antibiotics in terms of host cell immunity, mechanism of target cell resistance or tolerance, interaction requirements, mode of action, toxicity and side effect mechanisms (Cleveland et al., 2001; Balciunas et al., 2013). Various methods have been use like Agar well diffusion Method (S L. Ho and Won, 2009), Well dilution assay (Spanggaard et al., 2001) and Colony count assay (Godiosa et al., 1993). However, much of these previous studies were focused on to Lactobacillus live cells. Studies have revealed that Lactobacillus sp. has an antimicrobial activity against various pathogens. On the other hand, literature reveals that the secondary metabolites are released by the bacterial cell in growth medium or broth. Keeping these views in fact, supernatant was used inspite of the whole bacterial cell. (Amdekar et al., 2010).
Fhoula et al., support our study side to side with many researches in demonstrating the activity of bacteriocin produced by Lactic acid bacteria against G-ve and G+ve pathogen. Fhoula et al., isolated and screened the antimicrobial effect of a total of 119 LAB bacteria, Lactobacillus was one of the isolates had a strong antibacterial activity against plant and/or pathogenic bacteria of Stenotrophomonas maltophilia, Ps.savastanoi, Pantoea agglomerans, L. monocytogenes andthe food-borne Staph. aureus was recorded. (Fhoula et al., 2013). Gaamouche et al. 2014 examined the antimicrobial activity of bacteriocin produced by LAB bacteria against G+ve bacteria such as Listeria monocytogenes and Staphyloccocus aureus and also against G-ve bacteria such as Pseudomonas aeruginosa and E. coli. (Gaamouche et al., 2014). Doba and Saidi, isolated a 12 sample of bacteriocin-producing lactic acid bacteria from raw milk, two bacteriocin were effective against G-ve pathogen such as Pseudomonas aeruginosa and E. coli. (Doba and Saidi, 2015). All previous studies approved that many bacteriocin produced from gram positive bacteria have a strong antibacterial activity against both G+ve and G-ve pathogens.
The MIC of CAZ, IMP and MIN dramatically reduced when used in combination with Bacteriocin. The ΣFIC values highlighting the strong synergy between the three antimicrobials and Bacteriocin. In our study, the FIC indexes of CAZ, IMP and MIN combinations with the Bactriocin against S. maltophilia isolate were 0.249, 0.093 and 0.312, respectively. Confirming that a synergistic, not an additive relationship exists between them. Resistance of S. maltophilia to several antimicrobial agents restricts the choice of drugs for treating such infections. MDR strains further complicate the problem, as empiric antibiotic therapy usually remains inadequate or inappropriate in such patients. (Betriu et al., 2001; Valdezate et al., 2001).
The results of this study indicated high levels of MDR inS. maltophilia isolates agents CAZ, IMP and MIN. However, S. maltophilia has resistance mechanisms for these classes of agents. Although the choice of monotherapy or combination therapy is a controversial issue, several authors suggest combination treatment, especially in patients at risk (Poulos et al., 1995; Liaw et al., 2002; Falagas et al., 2008). Therefore, synergy testing may help determine the most appropriate combination in each special setting. Synergy testing in this study revealed that the most effective antibiotic combinations against S. maltophilia were bacteriocin plus imipenem, followed by Bacteriocin plus ceftazidime.
However, possibly a good option to combine the bacteriocins with other antibiotics. A synergy between bacteriocins and antimicrobial may potentiate each other killing activity, thus reducing the emergence of resistance to either antibiotics and bacteriocins. Besides, the combinations of antibiotics with bacteriocins can reduce the doses of antibacterial required to eradicate a pathogen, therefor minimizing the side effects accompanying the using of antibiotic. An example of adverse effects of polymyxin group of antibiotics is the nephrotoxicity (Mendes et al., 2009; Abdelraouf et al., 2012). One of the advantages of synergistic combinations of bacteriocins with antibiotics are decreases the funding associated with the synthesis and introducing of the more costly antibiotics. As well as, efficacious synergistic combinations of antibiotics and bacteriocins can extend the spectra of antibiotics, which may be beneficial in treating bacterial infections of unknown etiology. There are many methods to assessing antibacterial synergy in vitro Examples of such tests include the broth-based checkerboard assay, as well as agar-based screens such as E-tests (bioMérieux) to evaluate synergy (Sopirala et al., 2010; Soltani et al., 2012). However, there is a overall accord that the broth-based methods are more accurate than the agar-based assay. The fractional inhibitory concentration (FIC) index can calculated accurately by the checkerboard method (Orhan et al., 2005).
In one study, the most thoroughly investigated a combination between two types of bacteriocin lantibiotic, nisin at different concentrations ranging from 1.5 to 16 μg/ml, with the glycolipodepsipeptide ramoplanin “at concentrations ranging from 0.38 to 1.5 μg/ml” both of them target lipid II, resulting in synergistic activity against some MRSA strains tested (Brumfitt et al., 2002). LeBel et al. 2013 demonstrated that nisin interact synergistically when combined with the β-lactams antibiotics such as penicillin, ceftiofur or amoxicillin and as well as with tetracycline or streptomycin against veterinary pathogens such as Streptococcussuis (LeBel et al., 2013). A recent study established that a bactriocin-nisin was active against gram negative patogens like Pseudomonas aeruginosa biofilms when combined with polymyxins (Gellatly and Hancock, 2013; Field et al., 2016). It is particularly common in lungs of patients with cystic fibrosis and thus warrants further extensive research to target its biofilm forming and consequent pathogenic properties (Reen et al., 2016).
Additional advantages of bacteriocins including their synthesis in bacterial ribosome make them subjected to bioengineering strategies are the physiochemical properties such as improved solubility, tolerance to pH and resistant to proteases enzymes, thereby expanding their effectiveness as antibacterial. Unfortunately, the potential disadvantage of bacteriocins is a proteolytic digestion in GIT. However, this may be overcome by advances in encapsulation technologies or by giving them parenterally.
With respect to the clinical efficacy of bacteriocin antimicrobial combinations, the precise nature of physicochemical interactions, such as hydrophobic-hydrophobic or cationic-anionic interactions, between a proteinaceous bacteriocin and an antibiotic are likely to be important considerations when optimizing effective combinatorial therapy for use in vivo (Mathur et al., 2017).
Peter, 1995 define the Isobolography as an analysis provides a fundamental basis for assessing whether biological responses induced by mixtures of agents are greater, equal or smaller than would have been expected on the basis of the individual activities of the component agents and the concept of dose additivity (Peter, 1995). Drug combinations research expanded, led to increase the use of isobologram, a graph that was created and introduced by Loewe many years ago (Loewe, 1927, 1928). That graph, constructed on a coordinate system composed of the individual drug doses, commonly contains a straight “line of additivity” that is employed to distinguish additive from synergistic and antagonistic interactions. The construction of this graph is based on the assumption of a potency that relatively constant. The construction and use of the linear isobole discussed by Tallarida, put a set of points (dose pairs) that give a definite effect magnitude. In plots illustrated in our studied showed in Figures (7, 8 and 9) , each axis represents a serial of concentrations of one of the antibiotics or Bacteriocin, and the intercept values represent the MICs of the individual agents that produce the specified effect before and after combination. If testing shows that the specified effect of a combination is achieved by a dose pair that plots as a point below the isobole, this means super additively or synergism. In contrast, the point called sub additive and the dose pair plots as a point above the isobole line this mean that greater combination doses are needed to produce the specified effect. While if the dose pairs that experimentally locate on the line (or not significantly off the line) are termed additive, a situation that means no interaction between the two drugs. (Tallarida, 2012).
CONCLUSION
The effect of drug combinations have been studied for over hundred years by scientists. The advantages of combining drugs are well recognized, and activity in the area has increased dramatically thanks to the opportunities provided by the enhanced understanding of Systems Biology of disease. Isobolography and checkboard assay showed to a better method to determine the combination activity. The study revealed that the bacteriocins producing Lactobacillus acidophilus exposing strong inhibiting effect against Multidrug resistance S. maltophilia and it presented as an interesting alternative to antibiotics. Bacteriocins interacting synergistically with the antimicrobials used and the MICs of CAZ, IMP and MIN reduced dramatically. The net effect of antimicrobial combinations against S. maltophilia should further be evaluated in both the animal model and the clinical setting. It is noteworthy that in vitro studies lack the effects of host immune response. Nevertheless, antibiotic combinations may provide significant benefit over monotherapy in MDR pathogens such as S. maltophilia.
ACKNOWLEDGEMENTS
We would like to thank the Central Public Health lab. in Wasit province for providing L. acidophilus.
CONFLICT OF INTERESTS
This research is a personal non-profit work and there is no conflict of interest.
authors contribution
All authors contributed equally.
References





