Advances in Animal and Veterinary Sciences
Research Article
Identification of S.aureus and E.coli from Dairy Products Intended for Human Consumption
Aya Atef Kandil1, Mohamed Elhadidy2, Ahmed El-Gamal1, Maha Abdou Al-Ashmawy3*
1Animal Health Research Institute, Mansoura, Egypt; 2Department of Bacteriology, Mycology and Immunology, Faculty of Veterinary Medicine, Mansoura University, Egypt; 3Department of Food Hygiene and Control, Faculty of Veterinary Medicine, Mansoura University, Egypt.
Abstract | The present study was performed to determine the prevalence of S. aureus and E. coli in milk and some dairy products sold in Mansoura city, Egypt. A total two hundred samples including kariesh cheese (50 samples), market raw milk, market sterile milk, small scale yoghurt, large scale yoghurt, small scale ice cream, and large scale ice cream samples (25 samples from each product) were collected for microbiological examination. Recovered isolates were identified using an array of biochemical and serological tests for E. coli. Polymerase chain reaction (PCR) was applied to detect the nuc gene as a species-specific marker gene for S.aureus. Our result revealed that E. coli was observed in 86%, 60%, 88%,80%, 92% and 28% of kariesh cheese, market raw milk, small scale yoghurt, large scale yoghurt, small scale ice cream and large scale ice cream samples, respectively.None of the sterile milk samples was recorded for E. coli contamination. The prevalence of S. aureus was 92%, 80%, 36%, 100% and 40%, respectively. Likewise, none of the yoghurt large scale & sterile milk samples was recorded for S. aureus contamination. These findings highlighted the importance of periodical application of strict hygienic measures in dairy plants and markets for monitoring the public health hazards.
Keywords | E.coli, S.aureus, Milk, Dairy products.
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | May 29, 2018; Accepted | August 12, 2018; Published | October 04, 2018
*Correspondence | Maha Abdou Al-Ashmawy, Department of Food Hygiene and Control, Faculty of Veterinary Medicine, Mansoura University, Egypt; Email: mahaalashmawy@yahoo.com
Citation | Kandil AA, Elhadidy M, El-Gamal A, Al-Ashmawy MA (2018). Identification of S.aureus and E.coli from dairy products intended for human consumption. Adv. Anim. Vet. Sci. 6(11): 509-513.
DOI | http://dx.doi.org/10.17582/journal.aavs/2018/6.11.509.513
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2018 Kandil et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
The nutritional richness of milk is a goods ource of high biological value proteins and important vitamins and essential minerals (Pereira, 2014). Kareish cheese is one of the most popular cheese varieties consumed in Egypt especially in countryside due to its high protein, low fat and reasonable price (Metwalli, 2011). Likewise, yoghurt is considered as an excellent source of protein,vitamins, minerals and all the amino acids essential to heath (Mckinley, 2005). Ice cream is frequently considered as a ‘fun food,’ which is undeserving consideration. In reality, ice cream is a relatively well-balanced, healthy, easily digestible, and delicious food (Deosarkar et al., 2016). Milk & dairy products serves as very good medium for the growth of many microorganisms including pathogenic bacteria (Ruegg, 2003) accounting for 5% of all the incriminated foods in staphylococcal outbreaks, referring to Europe (Bianchiet al., 2014). Therefore milk & dairy products must be manufactured, stored and distributed according to good hygienic practices to avoid deterioration of the products and health hazards of consumers. Therefore, this study was carried out to highlight the microbiological status of milk and dairy products samples sold in Mansoura City, Egypt, specifically the prevalence of the most important food-borne pathogens S. aureus and E. coli.
Materials and methods
Collection and Preparation of Samples
Two hundred sampleswere collected for microbiological examination, including 50 milk samples (including 25 market raw milk samples and 25 market sterile milk samples), 50 kariesh cheese, 50 ice cream (including 25 small scale samples and 25 large scale samples), and 50 yoghurt (including 25 small scale samples and 25 large scale samples) (Table 1).
Table 1: Collection of the different samples for examination
| Area of collection | No. of samples | Products |
| Supermarkets in Mansoura city, Egypt | 50 | Kariesh cheese |
| Retail dairy shops in Mansoura city, Egypt | 25 | Raw market milk |
| Supermarkets in Mansoura city, Egypt | 25 | Sterile market milk |
| Retail dairy shops in Mansoura city, Egypt | 25 | Yoghurt small scale |
| Supermarkets in Mansoura city, Egypt | 25 | Yoghurt large scale |
| Ice cream shops in Mansoura city, Egypt | 25 | Ice cream small scale |
| Supermarkets in Mansoura city, Egypt | 25 | Ice cream large scale |
Microbial Examination
Identification and characterization of E. coli: Suspected isolates were confirmed by biochemical tests as published (Kreig and Holt, 1984). Serological identification was carried out by using rapid diagnostic E.coli antisera sets (Denka Seiken Co, Japan) for diagnosis of the Enteropathogenic types (Koki et al., 1996).
Identification and characterization of S. aureus: Colonies with typical morphology of S. aureus were streaked on Baired- parker agar medium for purification (APHA, ‘’American Public Health Association’’ 1992). Isolates were examined morphological identification by Gram`s stain (Cruickshank et al., 1975), biochemical tests (Arora, 2003) and identified by PCR for detection of nuc gene for detection of S. aureus gene (Sallam et al., 2015) using the primer sequence outlined in Table 2.
Table 2: Primer sets for PCR amplification of nuc gene specific for molecular identification of S.aureus.
| Target gene | Primer direction and sequence | Amplicon size (bp) | Source |
| Nuc |
F:5´-GTGCTGGCATATGTATGGCAATTG-3´ R:5´-CTGAATCAGCGTTGTCTTCGCTCCAA-3´ |
660 |
Statistical Analysis
Statistical analysis was performed by the (SAS, 2004) computer program using the general linear models (GLM). When the F-statistic was significant (P < 0.05), a mean separation was performed using the least significant difference (LSD) test.
Results
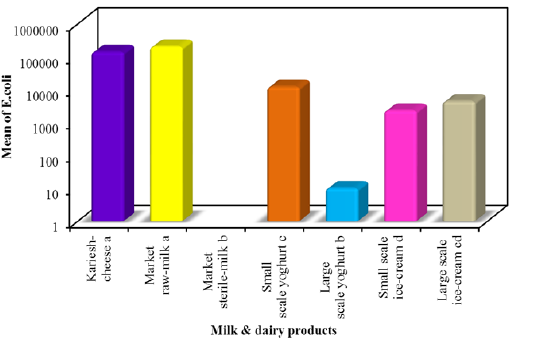
In this study E. coli was detected on EMB agar among all milk and dairy products with mean counts 1.4×105±1.9×104, 2.0×105 ± 4.4×104, 1.2×104± 2.0×103, 1.0×10 ± 2.1, 2.3×103 ± 4.9×102 and 4.4×103 ± 9.7×102 colony-forming units (CFU)/g or mlin kareish cheese, market raw milk, yoghurt small scale, yoghurt large scale, ice cream small scale and ice cream large scale samples, respectively. E. coli was not found in market sterile milk (Figure 1). The recognized E. coli strains from different dairy products biochemically identified E. coli isolates were sent to Food Analysis Center, at Faculty of veterinary medicine, Benha University in Egypt for serological identification and results of serological examination is outlined in Table 3. S.aureus was detected respectively with mean counts 2.4×105±4.6×104, 1.2×105± 2.2×104, 5.9×103± 1.1×103, 1.9×103± 1.9×102 and 1.1×103 ± 4.7×102 colony-forming units (CFU)/g or ml Figure 2 among tested kareish cheese, market raw milk, ice cream large scale, ice cream small scale, and yoghurt small scale samples respectively. Large scale yoghurt and market sterile milk was found negative for S. aureus. These results were confirmed by PCR detection of nuc gene as a species-specific marker gene for S.aureus (Figure 3). According to the results outlined it was obvious that large amount of dairy products available to the consumers that was not matching with the Egyptian standard (EOSQC, 2005) presented in Table 4 and Table 5.
Discussion
In the present study, the highest frequency of E. coli in kariesh cheese was 86% which lies within the range of 104 ≤
Table 3: Serological identification of representative isolates of E. coli (n=30) from milk & dairy products.
| Strain Characterization | Serodiagnosis | Positive Samples | Types of examined samples | |
| % | No. of isolated (30) | |||
|
ETEC |
O127: H6 | 6.66 | 2 |
Representative samples of Milk & Dairy products n=30 |
| O128: H2 | 13.33 | 4 | ||
|
EHEC
|
O111: H2 | 13.33 | 4 | |
| O26: H11 | 16.66 | 5 | ||
| O121: H7 | 3.33 | 1 | ||
| O103: H2 | 3.33 | 1 | ||
| EIEC | O124 | 6.66 | 2 | |
|
EPEC
|
O153: H21 | 3.33 | 1 | |
| O44: H18 | 6.66 | 2 | ||
| O6: H4 | 6.66 | 2 | ||
| O119: H6 | 6.66 | 2 | ||
| O15 | 3.33 | 1 | ||
| 90 | 27 | Total | ||
Table 4: Quality of dairy products according toEOSQC 1008-2005 for presence of E.coli on EMB agar.
| Unacceptable | Acceptable | Standard | No. of samples | Products | ||
| % | No. | % | No. | |||
| 86 | 43 | 14 | 7 | Free | 50 | Kariesh cheese |
| 60 | 15 | 40 | 10 | Free | 25 | Raw market milk |
| 0 | 0 | 100 | 25 | Free | 25 | Sterile market milk |
| 88 | 22 | 12 | 3 | Free | 25 | Yoghurt small scale |
| 80 | 20 | 20 | 5 | Free | 25 | Yoghurt large scale |
| 92 | 23 | 8 | 2 | Free | 25 | Ice cream small scale |
| 28 | 7 | 72 | 18 | Free | 25 |
Ice cream Large scale |
Table 5: Quality of dairy products according to EOSQC 1008-2005 for presence of S. aureus on Baired parker agar.
| Unacceptable | Acceptable | Standard | No. of samples | Product | ||||
| % | No. | % | No. | |||||
| 92 | 46 | 8 | 4 | Free | 50 | Kariesh cheese | ||
| 80 | 20 | 20 | 5 | <100 | 25 | Raw market milk | ||
| 0 | 0 | 100 | 25 | Free | 25 | Sterile market milk | ||
| 36 | 9 | 64 | 16 | Free | 25 | Yoghurt small scale | ||
| 0 | 0 | 100 | 25 | Free | 25 | Yoghurt large scale | ||
| 100 | 25 | 0 | 0 | Free | 25 | Ice cream small scale | ||
| 40 | 10 | 60 | 15 | Free | 25 | Ice cream large scale | ||
106. In Egypt, different reports highlighted the contamination of Kareish cheese with E. coli acting as a public health hazard (Ombarak et al., 2016) as this type of cheese is homemade and sold from door to door or sold in local markets (FAO, 1999). Different sources might be implicated in microbial contamination of kariesh cheese as starting with using poor quality of raw milk, processing under uncontrolled environments and selection of the inappropriate starter culture for the fermentation (Awad, 2016). Raw Milk serves as an excellent medium for the growth of many microorganisms and the highest frequency distribution of E. coli in raw milk was 60% lies within the range of 104 ≤ 106. Obviously, occurrence of E. coli in milk may arise from machines, manual milking and inferior quality of water (Chye et al., 2004). In this study, E.coli was not detected in sterile milk, suggesting their excellent sanitary quality as previously observed elsewhere (Gamal et al.,2015). The frequency distribution of E. coli in yoghurt was 88% in small scale which lies within the range of 103 ≤ 105and s 80% for large scale which lies within <103. Presence of E.c
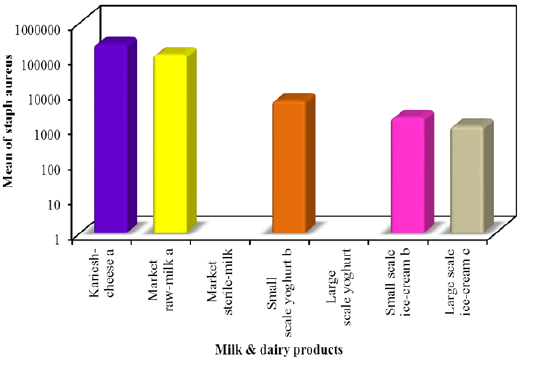
Figure 2: Statistical analytical results of S. aureus in milk and some dairy products on Baird parker medium.

Figure 3: Representative Agarose gel electrophoresis of PCR amplicons of the marker genes identified in S. aureus isolates from milk Dairy products samples. Amplified bands of the expected sizes of 660 bp for nuc gene.
-oli suggested post-pasteurization contamination of the product prior to or during packaging (Omola et al., 2014). Furthermore, these results suggested a general lack of cleanliness in handling and improper storage (Shojaei and Yadollahi, 2008). In ice cream, the frequency distribution of E. coli was 92% for small scale which lies within 103 ≤ 105 and in 28% in large scale which lies within 103 ≤ 104. Contamination can occur from different source include poor sanitary practices during processing and the use of contaminated components, mainly shell eggs and contaminated water (Walker et al., 1990).This difference in isolation rates might be attributed to different procedures used in packing and marketing of ice cream (Ambily and Beena, 2012). E. coli are harmless but some are known to be pathogenic bacteria, causing sever intestinal diseases in man (Kaper et al., 2004) and some strains are multiple drug resistant (MDR), this alarms the need for devising appropriate strategies to prevent the spread of resistance (Annal Selva- Malar et al., 2018). The highest frequency distribution of S. aureus in kariesh cheese was 92% whichlies within the range of 104 ≤ 107. Contamination can occur from different source include the poor quality of raw milk and processing in uncontrolled environments (Sameh, 2016). The highest frequency distribution of S. aureus in raw milk was 80% whichlies within the range of 104 ≤ 106. In sterile milk samples, S. aureuswasnot detected in all examined samples. Indicating that their excellent sanitary quality.The highest frequency distribution of S. aureus in small scale yoghurt was 36% which lies within the range of 103 ≤ 105. Presence of S. aureus in yoghurt usually indicates contamination from food handlers (Hussain, 2010). In the current study, the absence of bacterial contamination among large scale yoghurt suggested good sanitary quality. The highest frequency distribution of S. aureus in small scale ice cream was 40% which lies within the range of 103 ≤ 104, but in large scale ice cream cannot be detected. The isolation rates of S. aureus vary from investigator to another due to variable components used in preparation of ice cream (Reij and Den-Aantrekker, 2004). It was obvious that large amount of dairy products available to the consumers did not agree with Egyptian standard. This is referred to what degree of these products exposed to contamination during various stages of production.
Conclusion
Overall, these findings suggested that microbiological quality of raw milk and dairy products were highly contaminated with E.coli. and S.aureus. This indicates improper hygienic measures. Therefore, establishment of GMP “good manufacturing practice” and HACCP system in dairy plants and strict hygienic measures should be applied.
Acknowledgements
The authors would like to thank Pofessor Nazem.A.Shalaby, at Department of Animal Production, Faculty of Agriculture, Mansoura University for helping in statistical analysis of data.
Conflict of Interest
There is no conflict of interest.
Authors Contribution
AA and MA conceived and designed the study, performed the tests, analyzed and interpreted the data, and wrote the manuscript. ME and AE substantially contributed in analysis of the results and contributed to the writing of the manuscript. All authors read and approved the final manuscript.
References