Advances in Animal and Veterinary Sciences
Research Article
Effect of Molasses and Fermented Juice of Epiphytic Lactic Acid Bacteria on the Fermentation Characteristics and Nutrient Compositions of Cassava Leaves Silage
Achara Lukkananukool1, Chanathip Thammakarn1, Kanokrat Srikijkasemwat1, Min Aung2, Yin Yin Kyawt2*
1Department of Animal Production Technology and Fisheries, Faculty of Agricultural Technology, King Mongkut’s Institute of Technology Ladkrabang, Bangkok, 10520, Thailand; 2Departments of Animal Nutrition, University of Veterinary Science, Yezin, Nay Pyi Taw, 15013, Myanmar.
Abstract | Molasses and fermented juice of epiphytic lactic acid bacteria (FJLB) addition improved the quality of cassava leaves silage; however their effect depends on the types of forages ensiled. Thus, this study was aimed to compare the effect of molasses and FJLB additives on the fermentation characteristics and nutrient compositions of cassava leaves silage. Cassava leaves were chopped into pieces of 1-2 cm length before ensiling. The molasses 5% (w/w) and FJLB 1% (v/w) of fresh material were added as silage additives. As the measurement, the physical characteristics, chemical compositions, nutritive values, organic acid concentrations, lactic acid bacteria (LAB) count, volatile based nitrogen (VBN) and V-score of experimental silages were determined. In general, the physical characteristics and nutritive values of molasses and FJLB silages were not significantly different (p>0.05), however crude protein and fibre contents of FJLB silage were significantly higher (p<0.05) than molasses silage. Inversely, total carbohydrate and non fibre carbohydrate contents of molasses silage were also greater (p<0.05) than FJLB silage, resulted the higher feed intake in molasses silage. The acetic acid and propionic acid concentrations were greater (p<0.05) in molasses silage, whereas the higher (p<0.05) LAB count was found in FJLB silage. Regarding the indicators for good quality silage, VBN and V-score, both FJLB and molasses silages possessed the parameters for good quality silages. Thus, this experiment indicated that FJLB can be alternatively used as additive instead of using molasses in cassava leaves silage.
Keywords | Molasses, FJLB, Cassava leaves, Silage, V-score
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | July 20, 2018; Accepted | August 12, 2018; Published | September 05, 2018
*Correspondence | Yin Yin Kyawt, Departments of Animal Nutrition, University of Veterinary Science, Yezin, Nay Pyi Taw, 15013, Myanmar; Email: dr.yinyinkyawt81@gmail.com
Citation | Lukkananukool A, Thammakarn C, Srikijkasemwat K, Aung M, Kyawt YY (2018). Effect of molasses and fermented juice of epiphytic lactic acid bacteria on the fermentation characteristics and nutrient compositions of cassava leaves silage. Adv. Anim. Vet. Sci. 6(9): 388-394.
DOI | http://dx.doi.org/10.17582/journal.aavs/2018/6.9.388.394
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2018 Lukkananukool et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
The use of crop residues and agricultural by-products in animal feeding is a very common practice to ensure the feed supply for livestock production. Forage crops can be conserved through the fermentation process of ensiling, in which lactic acid bacteria (LAB) ferment water soluble carbohydrates (WSC) into organic acids (mainly lactic acid) in an anaerobic environment. As a result, pH is decreased and forage is conserved (Weinberg et al., 2001). Among the tropical crops, cassava is the most nutritional potential and it had been used as feed for livestock because of its remarkable carbohydrate and protein contents. Cassava leaves are the by-product of tuber production and rich in proteins (14–40%), vitamins, minerals, and carotenoids (Adewusi and Bradbury, 1993).
However, it is difficult to ensile the high protein legumes and tropical forage crop because of their low concentration of WSC and high buffering capacity (Mc.Donald et al., 1991; Titterton and Bareeba, 2000), and resulted in lower fermentation quality, intake, and digestibility (Niimi and Kawamura, 1998). Thus, the additives such as fermentation stimulants, fermentation inhibitors, aerobic deterioration inhibitors, nutrients and absorbents should be used to obtain the good quality silage.
Molasses, fermentation stimulant, should be added to protein rich forage to ensure successful fermentation (Pettersson, 1988) and their addition as a source of readily fermentable WSC increased lactic acid content and feeding quality (Yunus et al., 2000; Van Neikerk et al., 2007). It was also renowned that the silage quality of cassava and gliricidia tops residues (Man and Wiktorsson, 2002), and fermentative quality, feed intake and digestibility of Napier grass (Bureenok et al., 2012) were improved with the addition of molasses. The fermented juice of epiphytic lactic acid bacteria (FJLB) has been recommended as a silage additive for tropical grass silage (Bureenok et al., 2005a,b), and improved the silage quality of Napier grass (Bureenok et al., 2006), and the LAB counts and nutritive values of cassava leaves silage (Kyawt et al., 2014). Thus, as the report of Man and Wiktorsson, (2002) and Kyawt et al. (2014), both molasses and FJLB addition improved the quality of cassava leaves silage, however it is needed to compare the effect of FJLB and molasses on silage quality. On the other hand, all additives have ability to improve the quality of silage, however their effect varied and it depends on the types of forages ensiled. Thus, this study was aimed to compare the effect of molasses and FJLB additives on the fermentation characteristics and nutrient compositions of cassava leaves silage.
Materials and methods
Preparation of FJLB
The FJLB from napier Pak Chong1 grass was prepared 2 days prior to silage making. Each 200 g of fresh grass was macerated with 600 ml of distilled water using a blender. The macerate was then filtered through a sterilized double layer of cheesecloth and the filtrate was put into 600 ml flask. About 1% (w/v) of glucose was added into the filtrates and there were shaken well and kept in an incubator at 30°C for 2 days (Bureenok et al., 2005a).
Silage Making
Cassava leaves were immediately collected after harvesting in the field (Pak Chong, Nakhon Ratchasima Province, Thailand) and chopped into pieces of 1-2 cm length before ensiling. The FJLB 1% (v/w) and molasses 5% (w/w) of fresh material were added as silage additives and control silage was added with an equivalent amount of distilled water. Approximately 100 g fresh matter of treated crop then was packed into a plastic pouch in triplicate, and sealed with vacuum sealer (SQ202, Sharp, Co. Ltd, Japan). The bags were stored at room temperature and samples were taken at 21 days after ensiling for chemical analysis.
Measurements of Silage Quality
The physical characteristics of silages such as smell, taste, texture and color were assessed. For the determination of nutritional parameters, 20 g of fresh matter were extracted with 70 ml of distilled water and stored at 4°C for overnight. Then the extracts were filtered with filter paper and pH, ammonia nitrogen (NH3-N) and organic acid concentrations and V-score were measured. The pH of silage was measured by using a pH meter (F-23; Horiba, Tokyo, Japan). Ammonia nitrogen (NH3-N) and total nitrogen (TN) content were analyzed by using a steam distillation technique (Fross 2020 digester and Foss 2100 Kjeltec distillation unit, FOSS Analytical, Denmark) reported by JGFFSA (1994). Organic acid concentrations were determined by using High Performance Liquid Chromatography (Shim-pack SCR-102H, 300 mm × 8.0 mm id; column temperature, 40°C; flow rate, 0.8 mL/min, Shimadzu, Kyoto, Japan). The V-score was determined by method reported by Japan Grassland Farming Forage Seed Association (JGFFSA 1994).
Chemical Analysis
The rest silage materials are placed in the forced-air oven at 70˚C for 48 h to obtain the constant weight. After 48 h, all samples are immediately weighted and recorded it. All samples are ground with 1 mm sieve and analyzed for dry matter (DM), organic matter (OM), ether extract (EE) by the method described by (AOAC, 1990). Neutral detergent fibre (NDF) and acid detergent fibre (ADF) were analyzed according to the method of Goering and van Soest (1970). All feeds were analyzed for nitrogen (N) by using Kjeldahl method (Fross 2020 digester and Foss 2100 Kjeltec distillation unit) and crude protein (CP) was calculated as 6.25 x N (AOAC, 1990).
Table 1: Physical characteristics of cassava leaves ensiled with molasses and FJLB additives
| Descriptions | Treatment | ||
| Control | FJLB | Molasses | |
| pH |
5.37a |
5.07ab |
4.58b |
| Color | Yellowish green | Yellowish green | Yellowish green |
| Texture | Firm soft | Firm soft | Firm soft |
| Smell | Vinegar | Vinegar | Vinegar |
| Taste | Acidic | Acidic | Acidic |
FJLB: fermented juice of epiphytic lactic acid bacteria
Estimation of Nutritional Parameters of Silages
The nutritional parameters such as total carbohydrate (TC), non fibre carbohydrate (NFC), total digestible nitrogen (TDN), dry matter digestibility (DMD) and dry matter intake (DMI) were estimated by the formulas, which
Table 2: Chemical compositions of cassava leaves ensiled with molasses and FJLB additives
| Descriptions | Treatment (Means) | SEM | P value | ||
| Control | FJLB | Molasses | |||
| DM, % |
28.81b |
28.83b |
31.79a |
0.51 | 0.000 |
| OM, % DM | 94.53 | 94.59 | 95.76 | 0.64 | 0.738 |
| CP, % DM |
26.91a |
26.93a |
24.31b |
0.44 | 0.000 |
| NDF, % DM |
24.88b |
26.15a |
20.31c |
0.90 | 0.000 |
| ADF, % DM |
33.29a |
29.88ab |
26.71b |
1.14 | 0.028 |
| EE, % DM |
8.58a |
7.26b |
6.25c |
0.34 |
0.000 |
DM: dry matter, OM: organic matter, CP: crude protein, NDF: neutral detergent fibre, ADF: acid detergent fibre, EE: ether extract, FJLB: fermented juice of epiphytic lactic acid bacteria, SEM: standard error mean
All chemical compositions except DM are dry matter basis.
Different superscripts in the same row are significantly different at P value 0.05.
Table 3: Nutritive values of cassava leaves ensiled with molasses and FJLB additives
| Descriptions | Treatment (Means) | SEM | P value | ||
| Control | FJLB | Molasses | |||
| TC, % DM |
59.03b |
60.39b |
65.20a |
1.09 | 0.019 |
| NFC, % DM |
34.15b |
34.25b |
44.89a |
1.86 | 0.001 |
|
TDN, % DM |
64.53b |
66.92ab |
69.14a |
0.80 | 0.028 |
| DMD, % DM |
62.96b |
65.62ab |
68.09a |
0.89 | 0.029 |
|
DMI, % BW |
4.83b |
4.59c |
5.91a |
0.21 |
0.000 |
TC: total carbohydrate, NFC: non fibre carbohydrate, TDN: total digestible nitrogen, DDM: digestible dry matter, DMI: dry matter intake, BW: body weight, FJLB: fermented juice of epiphytic lactic acid bacteria, SEM: standard error mean
Different superscripts in the same row are significantly different at P value 0.05.
Table 4: Organic acid concentrations of cassava leaves ensiled with molasses and FJLB additives
| Descriptions | Treatment (Means) | SEM | P value | ||
| Control | FJLB | Molasses | |||
| Acetic/A, g/kg DM |
41.96b |
33.20b |
161.00a |
21.13 | 0.000 |
| Propionic/A, g/kg DM |
196.32b |
198.17b |
339.50a |
30.37 | 0.048 |
| Butyric/A, g/kg DM | 0.53 | 0.60 | 0.58 | 0.05 | 0.890 |
| Lactic/A, g/kg DM | 18.15 | 15.65 | 13.82 | 0.97 |
0.194 |
FJLB: fermented juice of epiphytic lactic acid bacteria, SEM: standard error mean
Different superscripts in the same row are significantly different at P value 0.05.
are expressed as followed.
TC = 100-(CP+EE+ASH) (NRC, 2001)
NFC = 100-(NDF+CP+EE+ASH) (NRC, 2001)
TDN (%DM) =87.84-(0.70*ADF) (Schmid et al., 1976)
DMD (% DM) = 88.9-(ADF*0.779) (NRC, 2001)
DMI (% of BW) =120/NDF (NRC, 2001)
Isolation of Lactic Acid Producing Bacteria
MRS (de Man, Rogosa and Sharpe) agar plates are used for the isolation of LAB. To distinguish acid-producing bacteria from other bacteria, 1% CaCO3, is added to the MRS-agar plates. Samples are incubated under anaerobic
conditions at 30˚C for 3 to 5 days. Colonies of acid-producing bacteria, identified by a clear zone around each colony, are randomly selected from MRS-agar plates and purified by replacing on MRS-agar plates. Colonies were counted as viable numbers of microorganisms, and are expressed as colony-forming unit per ml (log10 cfu/ml) (Kozaki et al., 1992).
Statistical Analysis
The data were subjected to the analysis of variance (ANOVA) and the significance of differences between means was compared by Duncan’s Multiple Range Test (DMRT) (Steel and Torrie, 1980) using SPSS (version 16.0) software.
Results
The physical characteristics of silages were presented in Table 1, in which no remarkably differences except pH were noted. The yellowish green color, firm soft texture, vinegar smell and acidic taste were generally observed in all silages. The pH value of FJLB silage was not significantly different (p>0.05) with control and molasses silages, wherein molasses silage had lower (p<0.05) pH value compared with control silage.
The significant variations (p<0.05) in chemical composition except OM were observed in Table 2. The higher (p<0.05) DM and lower (p<0.05) CP contents were observed in molasses silage compared with control and FJLB silages. The NDF content of experimental silages varied (p<0.05), in which the highest value was observed in FJLB silage and, followed by control and molasses silages. The ADF content of FJLB silage was not significantly different (p>0.05) with other two experimental silages, however that of molasses silage was lower (p<0.05) than control silage. The EE contents were significantly different (p<0.05) each other, which were ordered as control, FJLB and molasses silages.
The nutritive values of experimental silages were shown in Table 3. The TC and NFC contents of molasses silage were significantly higher (p<0.05) than those of control and FJLB silages. The TDN and DMD of FJLB were not significantly different (p>0.05) with both of control and molasses silages, in which molasses silage was higher (p<0.05) than control silage. For the estimation of DMI, the significant variations (p<0.05) were observed among the experimental silages, whereas the molasses silage was highest and followed by control and FJLB silages.
The organic acid concentrations of silages were described in Table 4. The acetic and propionic acid concentrations of molasses silage were significantly higher (p<0.05) than those of control and FJLB silages, while butyric and lactic acid concentrations were not different (p>0.05) among experimental silages.
The LAB count, VBN and V-score of experimental silages were shown in Figure 1A, 1B and 1C, respectively. The LAB count of FJLB silage was significantly higher (p<0.05) than those of control and molasses silages, which were not different (p>0.05). The VBN and V-score of FJLB silage were not statistically different (p>0.05) with control and molasses silages, however the lower (p<0.05) VBN and pH values, and the higher (p<0.05) V-score were observed in molasses silage compared with control silage.
Discussion
The significant changes on physical characteristics except pH were not noted in this experiment. The pH values of control and FJLB silages were greater than that of molas
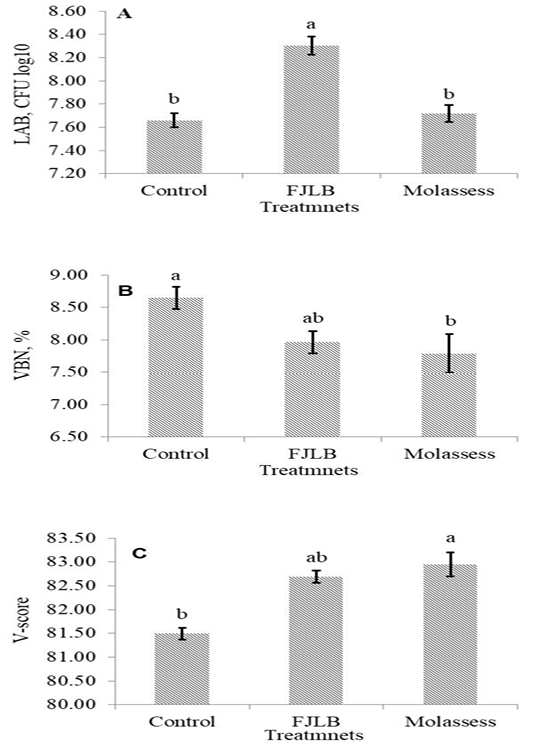 Figure 1: Lactic acid bacteria counts (1A), volatile based nitrogen (1B), and V-score (1C) of cassava leaves ensiled with molasses and FJLB additives
Figure 1: Lactic acid bacteria counts (1A), volatile based nitrogen (1B), and V-score (1C) of cassava leaves ensiled with molasses and FJLB additives
ses silage, which was very close with the acceptable rangefor good quality silage in the tropics (Bilal, 2009; Nhan et al., 2009). Molasses are rich in readily fermentable WSC and stimulates fermentation process, thereby reducing pH of silage (Pettersson, 1988; McDonald et al., 1991; Yunus et al., 2000; Van Neikerk et al., 2007). Man and Wiktorsson (2002) showed that molasses addition decreased pH content of cassava leaf silage. Addition of FJLB could not decrease the pH value of silage to the acceptable range. It is because low addition rate of FJLB, 1% (v/w), was used in the process of silage making, which could not supply the require population of LAB to reduce silage pH.
The improvement of DM content with the addition of molasses in this experiment is due to increase quality of fermentation (Harrison and Blauwiekel, 1994), thereby increasing population of LAB, improving quality of silage and avoiding the losses dry matter (McDonald et al., 1991). Man and Wiktorsson (2002) also reported that molasses addition increased DM content of cassava leaf silage. Inversely, the CP, NDF and ADF content were decreased with molasses addition, agreed with the report of Hill et al. (2001), and Huisden et al. (2009), they found that increase addition rates of molasses in silage making and long term fermentation reduced the CP, NDF and ADF content compared with control. McDonald et al. (1991) explained that molasses are silage additive which contain readily fermentable carbohydrates, resulted to decrease ammonia-N by stimulating fermentation. Moreover, molasses is a stimulant of silage and caused to increase analysis in cell wall (Baytok et al., 2005), thereby decreasing fibre content of silage. Huisden et al. (2009) also stated that the NDF concentration decreased after a long fermentation, which may be due to the enzymatic or acid hydrolysis of the cell wall fraction.
The calculations of nutritional parameters such as NFC, DMI, TDN and DMD related to NDF and ADF, respectively. The greater NFC and DMI in molasses silage are the result of the lower NDF content of it, and the higher TDN and DMD in FJLB and molasses silages are also the consequence of lower ADF content of those silage. These findings were consistent with the reports of (Bureenok et al., 2012); feed intake and digestibility of Napier grass were improved with the addition of molasses. Moreover, addition of FJLB also improved the silage quality of Napier grass (Bureenok et al., 2006), and the LAB counts and nutritive values of cassava leaves silage (Kyawt et al., 2014), thereby increasing digestibility and feed intake.
The acetic and propionic acid concentrations were enhanced with the addition of molasses in silage making, however no changes on butyric and lactic acid concentrations were observed in this experiment. Moreover, among organic acid concentrations, the propionic acid is the highest and the lowest is butyric acid. Acetic acid concentration is also higher than lactic acid in this experiment. This finding is consistent with the report of Shao et al. (2004) and Bureenok et al. (2005a,b), acetic acid is the primary fermentation acid in silages made from tropical grass.
The pH, LAB and lactic acid concentration were lowest in molasses silage, whereas acetic acid concentration was higher than other silages. On the other hand, the higher pH, LAB and lactic acid concentration were detected in the FJLB silage. The negative relation between pH and LAB, and positive relation between LAB and lactic acid concentration were generally observed in the process of silage making. Inversely, the positive relation between pH and LAB was found in this experiment. Moreover, the lactic acid concentration was lower than acetic acid concentration in all experimental silages, which is consistent with the report of Kyawt et al. (2014), the higher percentage of acetic acid than lactic acid was observed in the cassava leaves silage. There are two evident for these results. Firstly, the population of lactic acid bacteria had changed from homolactic to heterolactic during the changed fermentation (Shockey et al., 1988). Secondly, when the fresh material had low level of WSC content or even no more available carbohydrate, LAB were able to utilize lactic acid and produce more acetic acid (Lindgren et al., 1990).
The NH3-N or VBN works as an important indicator of proteolytic activity during the fermentation process. The VBN content of control silage is higher than FJLB and molasses silages, indicating degradation of the protein fraction of silage materials. The reason for this finding is due to the different CP contents of experimental silages, the highest CP contents were observed in control and FJLB silages, whereas the lowest is molasses silage. Haaland et al. (1982) reported that the higher protein content of feed provided the higher NH3 concentration as a result of the increasing proteolytic activity. However, it is not revealed in the FJLB silage, in which the higher CP and the lower VBN contents were observed. Kyawt et al. (2014) stated that NH3-N content in FJLB-treated silage was lower than that of control silage in ensiling of cassava leaves. It might be due to the higher LAB count found in FJLB silage. Thus, it would be suggested to conduct the further study for the clarification of the mechanism of LAB on the protein degradation of silage materials.
The VBN and V-score are the indicators for the determination of silage quality, which are negatively related. Thus, the good quality silage generally possessed the lower VBN and the higher V-score values. The over 80 points of V-score value indicated the good quality silage, whereas the range from 60 to 80 mean the normal silage, and below 60 points is for the low quality silage (JGFFSA, 1994). The FJLB and molasses silage possessed lower VBN value and higher V-score, over 82 points, indicating that they are good quality silages.
Conclusion
In general, the physical characteristics and nutritive values of molasses and FJLB silages were not significantly different, however the higher values for some parameters were observed in molasses silage. The acetic acid and propionic acid concentration were greater in molasses silage, whereas the higher LAB count was found in FJLB silage. Regarding the indicators for good quality silage, VBN and V-score, both FJLB and molasses silages possessed the parameters for good quality silages. Thus, this experiment indicated that FJLB can be alternatively used as additive instead of using molasses in cassava leaves silage.
AcknowledgementS
The authors would like to thanks Academic Melting Pot project from Faculty of Agricultural Technology, King Mongkut’s Institute of Technology Ladkrabang project for their funding assistance to conduct this experiment.
Conflict of interest
There is no conflict of interest.
AUTHOR’S CONTRIBUTION
AL, CT, KS, MA and YYK designed this experiment and, AL and YYK mainly carried out sample collection and silage making. AL, CT, KS and YYK analyzed the chemical compositions and silage quality. MA performed data analysis and interpretation. AL drafted the manuscript and CT, KS, MA and YYK completed the critical revision of the article. All authors read and approved the final version of manuscript.
References





