Advances in Animal and Veterinary Sciences
Research Article
Estimation of Penicillic acid in Poultry Edible Tissue (Gizzard)
Oday Sattar Abbas1*, Dalia Abdul-Kareem Abdul-Shaheed2
1Ibn Sina University of Medical and Pharmaceutical Sciences, Baghdad, Iraq; 2University of Baghdad, College of Veterinary Medicine, Baghdad, Iraq.
Abstract | Chicken is very sensitive to mycotoxin. Due to that, this study was planned to identify the presence of penicillic acid (PA) residues and quantum of this compound in 100 samples (Gizzard) were collected at random from various butcher shops in Baghdad Provincefor the period from October 2017 to January 2018 by using high-performance liquid chromatography (HPLC) for analyzed. The residues analysis showed that all poultry gizzard were positive for PA residues. The highest mean levels of PA residues in poultry gizzard samples recorded in January (26.46±0.05) as compared to those recorded in December (22.79±0.07), November (19.35±0.62) and October (15.87±0.03). The statistical analysis revealed that the levels of PA residues were significantly higher (P≤ 0.05) in January as compared to those recorded in October, November, and December.This study indicated that the presence of toxicogenic fungi in the poultry feeds increases the risk of mycotoxin food poison for animals and in turn for human beings and that we need to create awareness by the Ministry of Agriculture and Health on the work of preventive measures to reduce mycotoxins in poultry products levels.
Keywords | Penicillic acid, Poultry gizzard, High-performance liquid chromatography, mycotoxin, Penicillium spp
Editor | Kuldeep Dhama, Indian Veterinary Research Institute, Uttar Pradesh, India.
Received | June 12, 2018; Accepted | July 08, 2018; Published | August 12, 2018
*Correspondence | Oday Sattar Abbas, Ibn Sina University of Medical and Pharmaceutical Sciences, Baghdad, Iraq; Email: droddr7757@gmail.com
Citation | Abbas OS, Abdul-Shaheed DAK (2018). Estimation of penicillic acid in poultry edible tissue (gizzard). Adv. Anim. Vet. Sci. 6(9): 355-358.
DOI | http://dx.doi.org/10.17582/journal.aavs/2018/6.9.355.358
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2018 Abbas and Shaheed. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Fungi are ubiquitous to the environment and primarily saprophytic, using an organic material as a nutrient source for growth and reproduction. During the digestion, process fungi secrete enzymes into the nutrient source to break down complex compounds into simpler compounds. The digested nutrients are classified into two categories, primary and secondary metabolites (Ramesh, 2012). Mycotoxins are a structurally diverse group of mostly small molecular weight compounds that contaminate the whole food chain, from the harvested products to the plate of consumers (Danicke et al., 2011). Mycotoxins occur sporadically both seasonally and geographically, environmental factors such as light, temperature, pH and water activity (aw), either as single factors or in combination have an effect on mycotoxin production (Kalinina et al., 2017). Mycotoxin can enter the food supply in several ways, but these can be grouped into two general routes of contamination, direct or indirect contamination. Direct contamination occurs as a result of mold growth on the food material itself (Samuel, 2016). Almost all foods are susceptible to mold growth during some stage of production, processing, storage or transport, mold growth on foods that are to be consumed directly can result in indirect exposure to mycotoxin (Samuel, 2016). Penicillic acid, is a mycotoxins produced by many Penicillium and Aspergillus species, especially from Penicillium puberulum (Visagie et al., 2014, Alsberg and Black, 1913), P. aurantiogriseum, P. carneum, P. cyclopium, P. freii, P. melanoconidium, P. neoechinulatum, P. polonicum, P. radicicola, P. tulipae and P. viridicatum and in particular by the members of the Aspergillus ochraceus. It has been isolated from commercial maize and beans, peanuts, tree nuts, corn, animal feeds and on dry meat products (Frisvad et al., 2004). Penicillic acid has been reported to exert a variety of biological activities, including hepatotoxicity in experimental animals. It was also found to cause malignant, transplantable tumors in rats and mice, a hepatocarcinogen in some animal species, and has also been reported to affect the heart. Its demonstrates mutagenic and cytotoxic effects by inducing single-strand DNA breaks and inhibit DNA synthesis in CHO cells and also reported to irreversibly inactivate GDP-mannose dehydrogenase, interrupting the committed step in alginate biosynthesis (Visagie et al., 2014).
Due to a high consumption of poultry edible tissue, and an increased awareness of quality/residue free food concern among the consumers, lead to the investigation for the presence of mycotoxin residues in edible tissue. Therefore, this research was conducted to estimate the levels of PA residues in poultry tissue (Gizzard) sold in butcher shops in Baghdad Province, using high-performance liquid chromatography (HPLC).
MATERIALs AND METHODS
The research was conducted in coordination between thelaboratories of the Iraqi Ministry of Science and Technology and College of Veterinary Medicine/ University of Baghdad.
Chemicals, Reagents, and Standards
All reagents and solvents were HPLC or analytical grade. Penicillic acid (PA) standards from (Santa Crusz) Company, The analysis was performed on an Agilent 1200 Series HPLC with a diode array detector (DAD). The analytical column was an Agilent ZORBAX Eclipse Plus C18 2.1 mm×100 mm, 1.8μm. An Agilent 0.22 μm nylon Syringe Filter was used to filter the sample solution before HPLC.
Collection of Samples
It was taken 100 samples gizzard were collected at random sold in butcher shops in Baghdad Province for the period from October 2017 to January 2018. Samples were kept in cold ice during their transportationto the laboratory where they were kept at 4ᵒC until analysis.
Sample Extraction and Cleanup
In all samples were analyzed. All chickens were sacrificed by cervical dislocation, gizzard was collected, weighed, and frozen for subsequent PA analysis. Tissue samples were homogenized in distilled water with a blender, and the homogenate was sonicated for 1 min. with a sonicator. An equal volume of 3 N HC1 was added to the homogenate with mixing and the resulting solution was incubated at 80 ᵒC for 15 min. An equal volume of ethyl acetate was added, with mixing and centrifugation for 5 min. at 3,000 r/min. The ethyl acetate layer was collected and the homogenate extracted a second time. The two extracts were pooled and evaporated to dryness under a stream of nitrogen at 50ºC. After that residue was dissolved in 1 ml of acetonitrile: water (60:40, v/v) and washed twice with 2 ml of hexane.The extracts were analyzed by HPLC (Gary et al., 1981).
HPLC Conditions
The optimized instrumental conditions are summarized in Table 1.
Table 1: Instrumental conditions of HPLC
| Instrumental conditions | |
| Column | Agilent ZORBAX Eclipse Plus C18 2.1 mm×100 mm, 1.8μm |
| Flow rate | 1.0 ml/min |
| Injection volume | 20 μL |
| Column temperature | 35ºC |
| Detection wavelength | 254 nm |
|
Mobile phase |
Acetonitrile-Water (60:40) |
Statistical Analysis
The data were analyzed using one way ANOVA. Differences were considered significant at (P ≤ 0.05). SPSS (version 22) was used for statistical assessments.
RESULTS
The present data clearly demonstrated that all 100 samples obtained frombutcher shops in Baghdad Province during October 2017 to January 2018 were found to be contaminated with PA residues.
Table 2: Levels of Penicillic acid residues (ppm) in poultry gizzard between October 2017- January 2018 by HPLC
| Month (Mean±SE) | ||||
| Penicillic acid residues (ppm) | October | November | December | January |
|
15.87 ±0.03 *D |
19.35 ±0.62*C |
22.79 ±0.07*B |
26.46 ±0.05*A |
|
*The different letter within the raw are significantly different (p≤ 0.05).
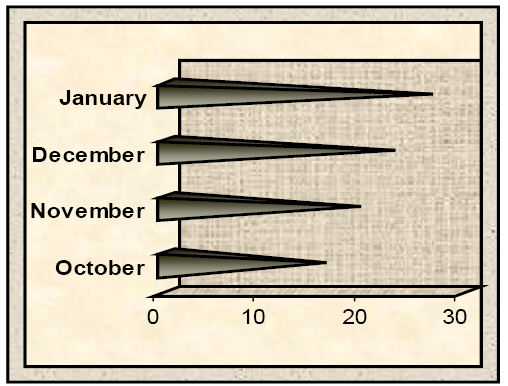
Figure 1: Levels of Penicillic acid residue (ppm) in poultry gizzard samples between October 2017- January 2018 by HPLC
The HPLC analyses showed that all poultry gizzard were positive for PA residues (Table 2). The highest mean levels of PA residues in poultry gizzard samples recorded in January (26.46±0.05) as compared to those recorded in December (22.79±0.07), November (19.35±0.62) and October (15.87±0.03).
Overall, statistical analysis revealed that The highest levels were recorded in January as compared to those recorded in December, November, and October in all gizzard samples and there were significant differences (P ≤ 0.05) between all study months (Figure 1).
DISCUSSION
The present research showed that all gizzard samples were positive for PA residue when analyses by the HPLC. The reason for increased the levels of PA in poultry gizzard samples in January followed December, November, and October due to in this month’s rain fell with poor storage of feed in a poultry farm and because that the molds are ubiquitous in nature and universally found where environmental conditions are conducive to mold growth. Because moulds are present in soil and plant debris, and are spread by wind currents, insects, and rain, they are frequently found in/on foods together with their associated mycotoxins (Habib et al., 2015), while (Garon et al., 2006) suggested that development of toxicogenic moulds in the animal feed and tissues is favoured by factors such as condensation, heating, leakage of rainwater, insect infection. Under certain environmental conditions, such as low temperature and high rainfall, grains are infected by fungi which produce mycotoxins (Sivakumar et al., 2014) and moldy growth on agricultural products results in the change in the texture, smell and taste of the infected foodstuff. This occurs due to excretion of enzymes and volatile compounds by the fungus. In addition to these, filamentous fungi also produce certain toxic secondary metabolites (mycotoxins) and these mycotoxins contaminate agricultural staples along the food production chain from the field, harvest, and transport to storage (Maria et al., 2014) .The excessive moisture in the field and in storage, temperature, humidity, drought, variations in harvesting practices and insect infestations are major environmental factors that determine the severity of mycotoxin contamination (Kalinina et al., 2017).
The results of current study was similar to (Gary et al., 1981) which determining PA residues in chicken tissues by liquid chromatographic (HPLC) after dosing orally of chickens with penicillic acid over a range of 50 to 550 mg/kg body weight resulted in detectable levels of the mycotoxin in gizzard muscle and contents, liver, kidney, heart, and intestinal contents and they proved that this method should use both for the rapid and sensitive detection of PA residues in poultry tissues.Another study (Talib, 2017) detected residual of PA in poultry kidney, meat, and liver. The positive percentages were 10% for kidney, 19.47% for muscles and 24.63% for livers.
ACKNOWLEDGMENTS
We appreciate the laboratories of the Iraqi Ministry of Science and Technology for its valuable cooperation in this research.
Conflict of interest
None of the authors have any conflict of interest to declare.
Authors Contribution
Oday Sattar Abbas: Plan of work, execution, HPLC analysis, and manuscript preparation.
Dalia Abdul-Kareem Abdul-Shaheed: Plan of work, technical assistance and manuscript preparation.
REFERENCES





