Research Journal for Veterinary Practitioners
Short Communication
Isolation of Escherichia coli from Poultry Liver and its Antibiogram Profile
Ameen-Ur-Rashid, Said Sajjad Ali Shah*, Mirza Ali Khan, Rafiullah, Anwar ali, Maleeha Anwar
Veterinary Research Institute, Peshawar, Khyber Pakhtunkhwa. Pakistan.
Abstract | Escherichia coli is a Gram-negative facultative anaerobic bacteria which cause serious threat to poultry industry by causing high morbidity and mortality. The aim of the present study was to determine the prevalence of E. coli in recent years (2014-2016) and antibiogram profile of E. coli isolates. Liver samples from different poultry farms were collected and processed for the confirmation of E. coli colonies. Antibiogram profile was also estimated for E. coli isolates by placing antibiotics disc and measuring zone of inhibition. Overall prevalence of E. coli was recorded as 35.1% while year wise prevalence was 29.2, 29.88 and 41.05% for year 2014, 2015 and 2016, respectively. Antibiogram profile of E. coli isolates showed that Gentamicin was highly sensitive in year 2014 and 2015 while ampicillin was least sensitive in these years while Colistin with 60% sensitivity was recorded highly sensitive in 2016 and Oxytetracyclin was highly resistant. Antibiogram profile of E. coli isolates showed that resistance developed to some antibacterial with passage of time while sensitivity of some antibacterial increased. Development of resistance might be due to the vigorous use of these antibiotics for control of diseases in poultry and also due to use of antibacterials as feed additives. Due to growing resistance to antibiotics it is highly recommended to decrease the unethical use of antibiotics to minimize the development of resistance strain of microbes in future.
Keywords | Escherichia coli, Poultry, Prevalence, Antibiogram, Zone of inhibition, Resistance
Editor | Muhammad Abubakar, National Veterinary Laboratories, Islamabad, Pakistan.
Received | March 12, 2017; Accepted | March 26, 2017; Published | March 27, 2017
*Correspondence | Said Sajjad Ali Shah, Veterinary Research Institute, Peshawar, Khyber Pakhtunkhwa. Pakistan; sajjadsheikh0695@gmail.com
Citation | Ameen-Ur-Rashid, Shah SSA, Khan MA, Rafiullah, Ali A, Anwar M (2017). Isolation of Escherichia coli from Poultry Liver and its Antibiogram Profile. Res. J. Vet. Pract. 5(1): 12-14.
DOI | http://dx.doi.org/10.17582/journal.rjvp/2017/5.1.12.14
ISSN (Online) | 2307-8316; ISSN (Print) | 2309-3331
Copyright © 2017 Ameen-Ur-Rashid et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
INTRODUCTION
Escherichia coli is a Gram-negative, rod shaped, facultative anaerobic bacterium that belongs to the Enterobacteriaceae family, which may cause a great hazard to poultry industry causing high mortality, loss of weight and reduction in egg production (Abd El Tawab et al., 2015). Escherichia coli infection is one of the serious problems that cause a great loss to the bird’s enterprises all over the word. Normally, it colonizes the intestinal tract of humans (Al-Arfaj et al., 2015) mammals and birds (Zanella et al., 2000) and cause disease under the influence of following factors, like poor and flawed ventilation, overcrowding due to less space and increase number of birds, hunger, dehydration, extremes of temperatures, increased mortality during rearing, decreased weight gain and condemnation of birds at the time of slaughter (Kaul et al., 1992) and also cause infection in immune suppressed hosts (Nataro and Kaper, 1998). Besides that, there are some E. coli strains originated from adapted pathogenic clones that cause diseases among healthy animals (Nataro and Kaper, 1998).
Pathogenic E. coli are associated with intestinal (Nataro and Kaper, 1998) extra intestinal infections in humans, like inflammation of urinary bladder and kidneys (Nakazato et al., 2009), septicemia, meningitis in infants (Welch, 2006). The pathogenic E. coli strains are also able to cause extra intestinal infections in other animals such as urinary infection (Peeters, 1994; Ling et al., 2001) pyometra in canines (Bjurstrom, 1993) and in case of birds it causes respiratory diseases (Kaper et al., 2004). Nowadays, E. coli is supposed to be the most important example of gram-negative bacterium that causes a large number of diseases because of the different pathogenicity mechanisms.
Colisepticemia is the most important disease caused by avian pathogenic Escherichia coli strains. This organism cause secondary bacterial infection in the upper respiratory tract of birds after primary viral infections such as the Newcastle disease, Infectious Bronchitis disease and Mycoplasma (Gross, 1991). These primary infections would increase the susceptibility of birds to Avian pathogenic E. coli strains by causing damage to the cilia of upper respiratory cells and expose these cells to ammonia and dust (Nakazato et al., 2009). The respiratory infection caused by avian pathogenic E. coli strains, further to the virus infection, is considered to be the initial step for colisepticemia development in birds (Gross, 1991). E. coli infection also called as aero sac disease; mostly it occurs among birds of two to twelve weeks of age with high mortality rates of about 20% (Dho-Moulin and Fairbrother, 1999).
These bacterial strains are a potential reservoir for antimicrobial resistance genes and play an important role in the ecology of antimicrobial resistance of bacterial populations. The intestinal fecal flora from poultry and other meat producing animals can also transfer antimicrobial resistance to human pathogens via food chain.
The aim of the current work was establish to record the prevalence of Escherichia coli infection in chickens and the antibiogram pattern against the isolated strains.
MATERIALS AND METHODS
Sample Collection
Total of 572 poultry liver samples were collected during the study period i.e. 2014-2016. Samples were either submitted by farmers or collected by the staff of the Pathology and Bacteriology section of Veterinary Research Institute, Peshawar.
Samples Processing For Growth
Samples were streaked aseptically on Tryptose and MacConkey’s agar plates as well as on blood agar for E. coli isolation. Plates were incubated at 37oC for 24-48 hours and then examined for the characteristic E. coli colonies. Pure colonies were of pink color on MacConkey’s agar, further identification was on the basis of cultural and morphological characteristics (Quinn et al., 2002).
In Vitro Antibiogram Of Isolates
E. coli isolates were streaked on Iso-Sensitest Agar (Oxoid) and different antibacterial discs were placed equivocally and incubated at 37oC for 24-48 hours. Sensitivity was determined by measuring zone of inhibition. Different antibacterial discs used were; Ampicillin (10µg), Chloramphenicol (30µg), Doxycyclin (30µg), Amoxycillin (30µg), Enrofloxacin (10µg), Gentamicin (10µg), Norflaxacin (10µg), Flumiquine (30µg), Ciproflaxacin (5µg), Oxytetracyclin (30µg), Sulphamethaxazole (25µg) and Colistin (10µg) (Oxoid, Basingstoke, UK).
Statistical Analysis
Data thus obtained was compiled in Microsoft Excel and analyzed for significance by using chi square-test through Statistix 8.1.
RESULTS AND DISCUSSION
Incidence Of E. coli In Liver
A total of 202 samples were found positive of E. coli out of 572 poultry liver samples. The overall prevalence of E. coli was 35.31% (Table 1). The year wise prevalence of E. coli was 29.20, 29.88 and 41.05% for the years 2014, 2015 and 2016, respectively and is non-significant statistically (P>0.05). Prevalence of E. coli was higher in 2016 while in 2014 and 2015 almost similar prevalence was recorded. This higher prevalence might be due to the poor management practices at poultry farms as E. coli is opportunistic pathogen. E. coli infection may occur as secondary infection when birds are immunosuppressed due to other diseases or environmental stress. Poor managmental practices and general hygienic conditions contribute to higher infection of E. coli (Zanella et al., 2000).
Table 1: Year wise occurrence of E. coli in poultry
|
Year |
Total number of samples tested |
Number of samples positive |
Occurrence (%) |
P-Value |
|
2014 |
113 |
33 |
29.20 |
0.08 |
|
2015 |
174 |
52 |
29.88 |
|
|
2016 |
285 |
117 |
41.05 |
|
|
Total samples processed |
572 |
202 |
35.3 |
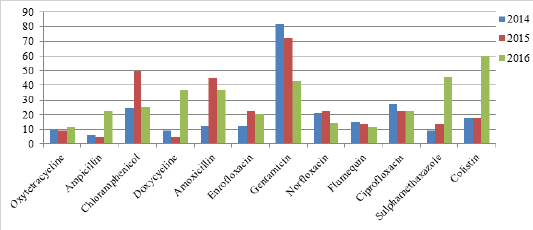
Antibiogram profile for E. coli isolates was almost similar in 2014 and 2015 with little fluctuation. Sensitivity to Gentamicin was higher i.e. 81 and 72% while ampicilin was recorded as least sensitive i.e. 6.6 and 4.54% in 2014 and 2015, respectively. Sensitivity to other antibacterials in 2014 were recorded as; Ciprofloxacin (27.2%), Chloramphenicol (24.23), Norfloxacin (21.2%), Colistin (18.1%), Flumequin (15.21%), Amoxicilin (12.14%), Enrofloxacin (12.14%), Oxytetracyclin (9.9%), Doxycycline (9.09%), Sulphamethazone (9.09%). In 2015 sensitivity was recorded as; Chloramphenicol (50%), Amoxicilin (45.45%), Ciprofloxacin (22.85%), Enrofloxacin (22.72%), Norfloxacin (22.7%), Colistin (18.2%), Sulphamethazone (13.63%), Flumequin (13.6%), Oxytetracyclin (9.09%), Doxycycline (2.54%). Colistin was recorded as highly sensitive in 2016 with 60% sensitivity while Oxytetracyclin was least sensitive (11.4%). Sensitivity to other antibacterials were recorded as; Sulphamethazone (45.71%), Gentamicin (42.85%), Amoxicilin (37.14%), Doxycyclin (37.14%), Chloramphenicol (25.71%), Ampicilin (22.85%), Ciprofloxacin (22.85%), Enrofloxacin (20%), Norfloxacin (14.28%), Flumequin (11.42%) (Figure 1).
Antibiogram profile of E. coli isolates showed that resistance developed to some antibacterial with passage of time while sensitivity of some antibacterial increased. Development of resistance might be due to the vigorous use of these antibiotics for control of diseases in poultry. In 2014, gentamicin was drug of choice with 81% sensitivity for colibacillosis infection in poultry but its sensitivity dropped to 42.85% in 2016. Antibiotics are used extensively in poultry and animals without any prescription which resulted in development of resistance strain of E. coli and thus antibiotic sensitivity decreased with passage of time. Results of the present study are in accordance with the findings of Catry et al. (2003) who reported that resistance developed with extensive use of antibiotics. Antibmicrobial agents are also used as feed additives, poultry feed etc. which also result in gradual increase of resistance to E. coli (Kang et al., 2005).
Due to vigorous use of antibiotics for control of diseases and in feed additives, resistance to the common antibiotics in the microbes developed. It is strongly recommended to decrease the unethical use of antibiotics to minimize the development of resistance strain of microbes in the future.
AUTHORS CONTRIBUTION
Rafiullah and AA conceived the idea of the manuscript. AUR and MA collected and processed the samples. SSAS analyzed the data and drafted the manuscript. All authors reviewed the draft.
REFERENCES